Magnetic Nanoparticles in Cancer Therapy and Diagnosis
- PMID: 32196144
- PMCID: PMC7482193
- DOI: 10.1002/adhm.201901058
Magnetic Nanoparticles in Cancer Therapy and Diagnosis
Abstract
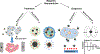
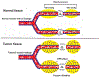
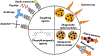
There is urgency for the development of nanomaterials that can meet emerging biomedical needs. Magnetic nanoparticles (MNPs) offer high magnetic moments and surface-area-to-volume ratios that make them attractive for hyperthermia therapy of cancer and targeted drug delivery. Additionally, they can function as contrast agents for magnetic resonance imaging (MRI) and can improve the sensitivity of biosensors and diagnostic tools. Recent advancements in nanotechnology have resulted in the realization of the next generation of MNPs suitable for these and other biomedical applications. This review discusses methods utilized for the fabrication and engineering of MNPs. Recent progress in the use of MNPs for hyperthermia therapy, controlling drug release, MRI, and biosensing is also critically reviewed. Finally, challenges in the field and potential opportunities for the use of MNPs toward improving their properties are discussed.
Keywords: cancer detection; cancer therapy; drug delivery; hyperthermia; magnetic nanoparticles.
© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

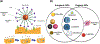









References
-
- Saghazadeh S, Rinoldi C, Schot M, Kashaf SS, Sharifi F, Jalilian E, Nuutila K, Giatsidis G, Mostafalu P, Derakhshandeh H, Adv. Drug Deliv.Rev. 2018, 127, 138; - PMC - PubMed
- Elkhoury K, Russell CS, Sanchez-Gonzalez L, Mostafavi A, Williams TJ, Kahn C, Peppas NA, Arab-Tehrany E, Tamayol A, Adv. Healthc. Mater. 2019, 8, 1900506. - PMC - PubMed
-
- Besenhard MO, Baber R, LaGrow AP, Mazzei L, Thanh NT, Gavriilidis A, Cryst. Eng. Comm. 2018, 20, 7082;
- Zhou X.-x., Liu J.-f., Jiang G.-b., Environ. Sci. Technol. 2017, 51, 3892. - PubMed
-
- Farzin A, Ahmadian M, Fathi M, Mater. Sci. Eng. C 2013, 33, 2251. - PubMed
-
- Tong R, Langer R, Cancer J. 2015, 21, 314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

