Real-World Evidence: Multicenter Efficacy and Toxicity Analysis of Nintedanib With Docetaxel as Second-Line Treatment in Mexican Patients With Advanced Lung Adenocarcinoma
- PMID: 32196388
- PMCID: PMC7113104
- DOI: 10.1200/JGO.19.00330
Real-World Evidence: Multicenter Efficacy and Toxicity Analysis of Nintedanib With Docetaxel as Second-Line Treatment in Mexican Patients With Advanced Lung Adenocarcinoma
Abstract
Purpose: The LUME-Lung 1 study has brought consistent evidence of the effective use of nintedanib in lung adenocarcinoma as a second line of treatment; however, differences among ethnicities have been found in some studies.
Methods: This was a retrospective review among 21 medical centers of 150 patients with a confirmed diagnosis of lung adenocarcinoma, included in a compassionate use program of nintedanib from March 2014 to September 2015. The current study aimed to analyze the effectiveness of nintedanib in combination with docetaxel in the Mexican population, using progression-free survival rate and the best objective response to treatment by RECIST 1.1 as a surrogate of effectiveness. In addition, we examined the toxicity profile of our study population as a secondary end point.
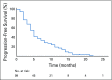
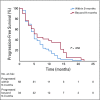
Results: After exclusion criteria, only 99 patients met the criteria for enrollment in the current study. From the total study population, 53 patients (53.5%) were male and 46 (46.5%) were female, with an average age of 60 years and stage IV as the most prevalent clinical stage at the beginning of the compassionate use program. A total of 48 patients (48.5%) had partial response; 26 (26.3%), stable disease; 4 (4%), complete response; and 16 (16.2%), progression; and 5 (5%) were nonevaluable. We found a median progression-free survival of 5 months (95% CI, 4.3 to 5.7 months). The most common grade 3 or 4 adverse reactions were fatigue (14%) and diarrhea (13%).
Conclusion: Nintedanib, as part of a chemotherapy regimen, is an effective option with an acceptable toxicity profile for advanced lung adenocarcinoma after first-line treatment progression.
Conflict of interest statement
Jeronimo Rafael Rodríguez-Cid
Saul Campos-Gomez
Vanessa García-Montes
Manuel Magallanes-Maciel
Iván Romarico González-Espinoza
Juan Paulo Ceja García
Juan Carlos Cázarez-Price
Carlos Jose Zuloaga-Fernandez
David Suárez-García
Raquel Gerson-Cwilich
Leticia Vázquez-Cortés
Alicia Acosta-Espinoza
Jorge Arturo Alatorre-Alexander
No other potential conflicts of interest were reported.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- International Agency for Research on Cancer Global Cancer Observatory. http://globocan.iarc.fr/
-
- Instituto Nacional de Estadística y Geografía Estadísticas a propósito del día mundial contra el cáncer (4 de febrero) http://www.beta.inegi.org.mx/contenidos/saladeprensa/aproposito/2018/can...
-
- National Cancer Institute Non-Small Cell Lung Cancer Treatment (PDQ) Bethesda, MD, National Institutes of Health; 2019
-
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous