Novel truncating mutations in CTNND1 cause a dominant craniofacial and cardiac syndrome
- PMID: 32196547
- PMCID: PMC7372553
- DOI: 10.1093/hmg/ddaa050
Novel truncating mutations in CTNND1 cause a dominant craniofacial and cardiac syndrome
Abstract
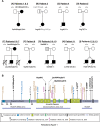
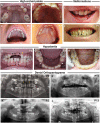
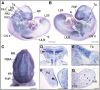
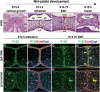
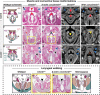
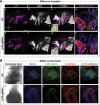
CTNND1 encodes the p120-catenin (p120) protein, which has a wide range of functions, including the maintenance of cell-cell junctions, regulation of the epithelial-mesenchymal transition and transcriptional signalling. Due to advances in next-generation sequencing, CTNND1 has been implicated in human diseases including cleft palate and blepharocheilodontic (BCD) syndrome albeit only recently. In this study, we identify eight novel protein-truncating variants, six de novo, in 13 participants from nine families presenting with craniofacial dysmorphisms including cleft palate and hypodontia, as well as congenital cardiac anomalies, limb dysmorphologies and neurodevelopmental disorders. Using conditional deletions in mice as well as CRISPR/Cas9 approaches to target CTNND1 in Xenopus, we identified a subset of phenotypes that can be linked to p120-catenin in epithelial integrity and turnover, and additional phenotypes that suggest mesenchymal roles of CTNND1. We propose that CTNND1 variants have a wider developmental role than previously described and that variations in this gene underlie not only cleft palate and BCD but may be expanded to a broader velocardiofacial-like syndrome.
© The Author(s) 2020. Published by Oxford University Press.
Figures



References
-
- Ghoumid J., Stichelbout M., Jourdain A.S., Frenois F., Lejeune-Dumoulin S., Alex-Cordier M.P., Lebrun M., Guerreschi P., Duquennoy-Martinot V., Vinchon M. et al. (2017) Blepharocheilodontic syndrome is a CDH1 pathway-related disorder due to mutations in CDH1 and CTNND1. Genet. Med., 19, 1013–1021. - PubMed
-
- Cox L.L., Cox T.C., Moreno Uribe L.M., Zhu Y., Richter C.T., Nidey N., Standley J.M., Deng M., Blue E., Chong J.X. et al. (2018) Mutations in the epithelial cadherin-p120-catenin complex cause Mendelian non-Syndromic cleft lip with or without cleft palate. Am. J. Hum. Genet., 102, 1143–1157. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

