Lamotrigine add-on therapy for drug-resistant focal epilepsy
- PMID: 32196639
- PMCID: PMC7083514
- DOI: 10.1002/14651858.CD001909.pub3
Lamotrigine add-on therapy for drug-resistant focal epilepsy
Update in
-
Lamotrigine add-on therapy for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2023 Dec 11;12(12):CD001909. doi: 10.1002/14651858.CD001909.pub4. Cochrane Database Syst Rev. 2023. PMID: 38078494 Free PMC article.
Abstract
Background: This is an updated version of the Cochrane Review previously published in 2016. Epilepsy is a common neurological disorder, affecting 0.5% to 1% of the population. For nearly 30% of these people, their epilepsy is resistant to currently available drugs. Pharmacological treatment remains the first choice to control epilepsy. Lamotrigine is one of the newer antiepileptic drugs. Lamotrigine, in combination with other antiepileptic drugs (add-on), can reduce seizures, but with some adverse effects.
Objectives: To determine the effects of lamotrigine on (1) seizures, (2) adverse-effect profile, and (3) cognition and quality of life, compared to placebo, when used as an add-on treatment for people with drug-resistant focal epilepsy.
Search methods: For the latest update of the review, we searched the following databases on 9 March 2020: Cochrane Register of Studies (CRS Web), MEDLINE (Ovid, 1946 to March 06, 2020). CRS Web includes randomized or quasi-randomized, controlled trials from PubMed, EMBASE, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups including Epilepsy. No language restrictions were imposed.
Selection criteria: Randomised placebo-controlled trials of people with drug-resistant focal epilepsy of any age, in which an adequate method of concealment of randomisation was used. The studies were double-, single- or unblinded, placebo-controlled. For cross-over studies, the first treatment period was treated as a parallel trial. Eligible participants were adults or children with drug-resistant focal epilepsy.
Data collection and analysis: For this update, two review authors independently assessed the trials for inclusion, and extracted data. Outcomes included 50% or greater reduction in seizure frequency, treatment withdrawal (any reason), adverse effects, effects on cognition and quality of life. Primary analyses were by intention-to-treat. Sensitivity best- and worse-case analyses were undertaken to account for missing outcome data. Pooled risk ratios (RRs) with 95% confidence intervals (95% Cls) were estimated for the primary outcomes of seizure frequency and treatment withdrawal. For adverse effects, we calculated pooled RRs and 99% Cls.
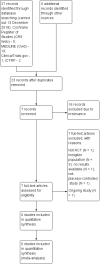
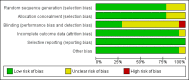
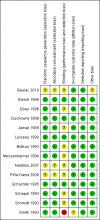
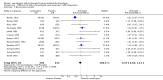
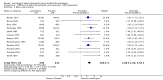
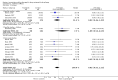
Main results: We did not identify any new studies for this update, therefore, the results and conclusions are unchanged. In previous updates of this review, the authors found five parallel add-on studies, eight cross-over studies in adults or children with drug-resistant focal epilepsy, and one parallel add-on study with a responder-enriched design in infants. In total, these 14 studies included 1806 eligible participants (38 infants, 199 children, 1569 adults). Baseline phases ranged from four to 12 weeks; treatment phases from eight to 36 weeks. Overall, 11 studies (1243 participants) were rated as having low risk of bias, and three (697 participants) had unclear risk of bias due to lack of reported information around study design. Effective blinding of studies was reported in four studies (563 participants). The overall risk ratio (RR) for 50% or greater reduction in seizure frequency was 1.80 (95% CI 1.45 to 2.23; 12 trials, 1322 participants (adults and children); moderate-certainty evidence) indicating that lamotrigine was significantly more effective than placebo in reducing seizure frequency. The overall RR for treatment withdrawal (for any reason) was 1.11 (95% CI 0.91 to 1.37; 14 trials; 1806 participants; moderate-certainty evidence). The adverse events significantly associated with lamotrigine were: ataxia, dizziness, diplopia (double vision), and nausea. The RR of these adverse effects were as follows: ataxia 3.34 (99% Cl 2.01 to 5.55; 12 trials; 1525 participants; high-certainty evidence); dizziness 2.00 (99% Cl 1.52 to 2.64;13 trials; 1768 participants; moderate-certainty evidence); diplopia 3.79 (99% Cl 2.15 to 6.68; 3 trials, 944 participants; high-certainty evidence); nausea 1.81 (99% Cl 1.22 to 2.68; 12 studies,1486 participants; moderate-certainty evidence). The limited data available precluded any conclusions about effects on cognition and quality of life. No important heterogeneity between studies was found for any of the outcomes. Overall, we assessed the evidence as high to moderate certainty, due to incomplete data for some outcomes.
Authors' conclusions: Lamotrigine as an add-on treatment for drug-resistant focal seizures appears to be effective in reducing seizure frequency, and seems to be fairly well-tolerated. However, the trials were of relatively short duration and provided no evidence for the long term. Further trials are needed to assess the long-term effects of lamotrigine, and to compare lamotrigine with other add-on drugs.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MP: none known. SR: none known. RB: none known AGM: A consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is part funded by the Applied Research Collaboration North West Coast (ARC NWC).
Figures








Update of
-
Lamotrigine add-on for drug-resistant partial epilepsy.Cochrane Database Syst Rev. 2016 Jun 22;2016(6):CD001909. doi: 10.1002/14651858.CD001909.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Mar 20;3:CD001909. doi: 10.1002/14651858.CD001909.pub3. PMID: 27329345 Free PMC article. Updated.
Similar articles
-
Lamotrigine add-on therapy for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2023 Dec 11;12(12):CD001909. doi: 10.1002/14651858.CD001909.pub4. Cochrane Database Syst Rev. 2023. PMID: 38078494 Free PMC article.
-
Lamotrigine add-on for drug-resistant partial epilepsy.Cochrane Database Syst Rev. 2016 Jun 22;2016(6):CD001909. doi: 10.1002/14651858.CD001909.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Mar 20;3:CD001909. doi: 10.1002/14651858.CD001909.pub3. PMID: 27329345 Free PMC article. Updated.
-
Gabapentin add-on treatment for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2021 Jan 12;1(1):CD001415. doi: 10.1002/14651858.CD001415.pub4. Cochrane Database Syst Rev. 2021. PMID: 33434292 Free PMC article.
-
Lamotrigine add-on therapy for drug-resistant generalised tonic-clonic seizures.Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD007783. doi: 10.1002/14651858.CD007783.pub3. Cochrane Database Syst Rev. 2020. PMID: 32609387 Free PMC article.
-
Pregabalin add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2019 Jul 9;7(7):CD005612. doi: 10.1002/14651858.CD005612.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Mar 29;3:CD005612. doi: 10.1002/14651858.CD005612.pub5. PMID: 31287157 Free PMC article. Updated.
Cited by
-
Antiepileptic Strategies for Patients with Primary and Metastatic Brain Tumors.Curr Treat Options Oncol. 2024 Mar;25(3):389-403. doi: 10.1007/s11864-024-01182-8. Epub 2024 Feb 14. Curr Treat Options Oncol. 2024. PMID: 38353859 Free PMC article. Review.
-
Lamotrigine add-on therapy for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2023 Dec 11;12(12):CD001909. doi: 10.1002/14651858.CD001909.pub4. Cochrane Database Syst Rev. 2023. PMID: 38078494 Free PMC article.
-
Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens-Johnson Syndrome-A Narrative Review of the Existing Literature.J Clin Med. 2025 Jun 10;14(12):4103. doi: 10.3390/jcm14124103. J Clin Med. 2025. PMID: 40565849 Free PMC article. Review.
-
Preliminary analysis of the effect of vagus nerve stimulation in the treatment of children with intractable epilepsy.World J Clin Cases. 2020 Dec 6;8(23):5918-5925. doi: 10.12998/wjcc.v8.i23.5918. World J Clin Cases. 2020. PMID: 33344590 Free PMC article.
-
The effectiveness of antiepileptic drug treatment in glioma patients: lamotrigine versus lacosamide.J Neurooncol. 2021 Aug;154(1):73-81. doi: 10.1007/s11060-021-03800-z. Epub 2021 Jul 1. J Neurooncol. 2021. PMID: 34196916 Free PMC article.
References
References to studies included in this review
Baulac 2010 {published data only}
-
- Baulac M, Leon T, O'Brien TJ, Whalen E, Barrett J. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial‐onset seizures. Epilepsy Research 2010; Vol. 91, issue 1:10‐9. - PubMed
Binnie 1989 {published data only}
-
- Binnie CD, Debets RM, Engelsman M, Meijer JW, Meinardi H, Overweg J, et al. Double‐blind crossover trial of lamotrigine (Lamictal) as add‐on therapy in intractable epilepsy. Epilepsy Research 1989;4:222‐9. - PubMed
Boas 1996 {published and unpublished data}
-
- Boas J, Dam M, Friis ML, Kristensen O, Pedersen B, Gallagher J. Controlled trial of lamotrigine (Lamictal) for treatment‐resistant partial seizures. Acta Neurologica Scandinavica 1996;94:247‐52. - PubMed
Duchowny 1999 {published data only}
-
- Duchowny M, Pellock JM, Graf WD, Billard C, Gilman J, Casale E, et al. A placebo‐controlled trial of lamotrigine add‐on therapy for partial seizures in children. Neurology 1999;53(8):1724‐31. - PubMed
Jawad 1989 {published data only}
-
- Jawad S, Richens A, Goodwin G, Yuen WC. Controlled trial of lamotrigine (lamictal) for refractory partial seizures. Epilepsia 1989;30(3):356‐63. - PubMed
Loiseau 1990 {published data only}
-
- Loiseau P, Yuen AW, Duche B, Menager T, Arne‐Bes MC. A randomised double‐blind placebo‐controlled crossover add‐on trial of lamotrigine in patients with treatment‐resistant partial seizures. Epilepsy Research 1990;7:136‐45. - PubMed
Matsuo 1993 {published data only}
-
- Matsuo F, Bergen D, Faught E, Messenheimer JA, Dren AT, Rudd GD, et al. Placebo‐controlled study of the efficacy and safety of lamotrigine in patients with partial seizures. US Lamotrigine Protocol 0.5 Clinical Trial Group. Neurology 1993;43:2284‐91. - PubMed
Messenheimer 1994 {published data only}
-
- Messenheimer J, Ramsay RE, Willmore LJ, Leroy RF, Zielinski JJ, Mattson R, et al. Lamotrigine therapy for partial seizures: a multicenter, placebo‐controlled, double‐blind, cross‐over trial. Epilepsia 1994;35(1):113‐21. - PubMed
Naritoku 2007 {published data only}
-
- Naritoku DK, Warnock CR, Messenheimer JA, Borgohain R, Evers S, Guekht AB, et al. Lamotrigine extended‐release as adjunctive therapy for partial seizures. Neurology 2007;69:1610‐8. - PubMed
Piña‐Garza 2008 {published data only}
-
- Piña‐Garza JE, Levisohn P, Gucuyener K, Mikati MA, Warnock CR, Conklin HS, et al. Adjunctive lamotrigine for partial seizures in patients aged 1 to 24 months. Neurology 2008;70:2099‐108. - PubMed
Schachter 1995 {published and unpublished data}
-
- Schachter SC, Leppik IE, Matsuo F, Messenheimer JA, Faught E, Moore EL, et al. Lamotrigine: a six‐month, placebo‐controlled, safety and tolerance study. Journal of Epilepsy 1995;8:201‐9.
Schapel 1993 {published and unpublished data}
Schmidt 1993 {published and unpublished data}
-
- Schmidt D, Ried S, Rapp P. Add‐on treatment with lamotrigine for intractable partial epilepsy: a placebo‐controlled, cross‐over trial. Epilepsia 1993;34 Suppl(2):66.
Smith 1993 {published and unpublished data}
-
- Smith D, Baker GA, Davies G, Dewey M, Chadwick DW. Outcomes of add‐on treatment with lamotrigine in partial epilepsy. Epilepsia 1993;34(2):312‐22. - PubMed
References to studies excluded from this review
Berg 2015 {published data only}
-
- Berg M, Diaz F, Bolger P, Dworetzky B, Elder E, Gidal B. Equivalence among generic AEDs (equigen)‐single‐dose study. Epilepsy Currents 2015;15(Suppl 1):321, Abstract no: 2.287.
Berg 2017 {published data only}
-
- Berg M, Welty TE, Gidal BE, Diaz FJ, Krebill R, Szaflarski JP. Bioequivalence between generic and branded lamotrigine in people with epilepsy: the EQUIGEN randomized clinical trial. JAMA Neurology 2017; Vol. 74, issue 8:919‐26. [DOI: 10.1001/jamaneurol.2017.0497; PUBMED: 28654954] - DOI - PMC - PubMed
Biton 2010 {published data only}
-
- Biton V, Memmo J, Shukla R, Lee YY, Poverennova I, Demchenko V, et al. Adjunctive lamotrigine XR for primary generalized tonic‐clonic seizures in a randomized, placebo‐controlled study. Epilepsy & Behavior 2010; Vol. 19, issue 3:352‐8. - PubMed
Biton 2013 {published data only}
-
- Biton V, Shneker BF, Naritoku D, Hammer AE, Vuong A, Caldwell PT, et al. Long‐term tolerability and safety of lamotrigine extended‐release: pooled analysis of three clinical trials. Clinical Drug Investigation 2013;33(5):359‐64. - PubMed
Brzakovic 2012 {published data only}
-
- Brzakovic BB, Vezmar Kovacevic SD, Vucicevic KM, Miljkovic BR, Martinovic ZJ, Pokrajac MV, et al. Impact of age, weight and concomitant treatment on lamotrigine pharmacokinetics. Journal of Clinical Pharmacy & Therapeutics 2012;37(6):693‐7. - PubMed
Carignani 2004 {published data only}
-
- Carignani MA, Matassa JC. Controlled trial of lamotrigine for the treatment of refractory partial seizures. Epilepsia 2004;45 Suppl 3:135‐6.
Carignani 2006 {published data only}
-
- Carignani M, Matassa J, Rosso D. Controlled trial of lamotrigine for the treatment of refractory partial seizures. Epilepsia 2006;47 Suppl 3:124.
Chung 2009 {published data only}
Contin 2016 {published data only}
-
- Contin M, Alberghini L, Candela C, Benini G, Riva R. Intrapatient variation in antiepileptic drug plasma concentration after generic substitution vs stable brand‐name drug regimes. Epilepsy Research 2016;122:79‐83. - PubMed
Cramer 2013 {published data only}
-
- Cramer JA, Sapin C, Francois C. Indirect comparison of clobazam and other therapies for Lennox‐Gastaut Syndrome. Acta Neurologica Scandinavica 2013;128(2):91‐9. - PubMed
French 2012 {published data only}
-
- French JA, Hammer AE, Vuong A, Messenheimer JA. Analysis of three lamotrigine extended‐release clinical trials: comparison of pragmatic ITT and LOCF methodologies. Epilepsy Research 2012;101(1‐2):141‐7. - PubMed
Frith 2015 {published data only}
-
- Frith R, Bergin P, Jayabal J, Korosec M, Rodriguez‐Leyva I, Alkhidze M. The EpiNet‐first trials now recruiting patients with newly diagnosed epilepsy. Journal of the Neurological Sciences 2015;357:e144.
Girolineto 2012 {published data only}
-
- Girolineto BM, Alexandre Junior V, Sakamoto AC, Pereira LR. Interchangeability among therapeutic equivalents of lamotrigine: evaluation of quality of life. Brazilian Journal of Pharmaceutical Sciences 2012;48(1):95‐102.
Hammer 2008 {published data only}
-
- Hammer AE, Vuong A, Kustra R, Messenheimer JA. Pragmatic intent‐to‐treat analysis for lamotrigine extended release adjunctive therapy in patients with intractable partial seizures. Epilepsia 2008;49(Suppl 7):100.
Hartung 2012 {published data only}
Helmstaedter 2013 {published data only}
-
- Helmstaedter C, Witt JA. The longer‐term cognitive effects of adjunctive antiepileptic treatment with lacosamide in comparison with lamotrigine and topiramate in a naturalistic outpatient setting. Epilepsy & Behaviour 2013;26(2):182‐7. - PubMed
IRCT2013021211560N3 2013 {published data only}
-
- IRCT2013021211560N3. Epilepsy management in elderly. http://www.irct.ir/searchresult.php?id=11560&number=3 2013.
Kang 2012 {published data only}
-
- Kang HC, Hu Q, Liu XY, Xu F, Li X, Liu ZG, et al. Efficacy and safety of the combined therapy of valproic acid and lamotrigine for epileptics. Zhonghua yi xue za zhi 2012;92(17):1174‐8. - PubMed
Lee 2018 {published data only}
-
- Lee BI, No SK, Yi S‐D, Lee HW, Kim OJ, Kim SH. Unblinded, randomized multicenter trial comparing lamotrigine and valproate combination with controlled‐release carbamazepine monotherapy as initial drug regimen in untreated epilepsy. Seizure 2018;55:17‐24. - PubMed
Mintzer 2018 {published data only}
-
- Mintzer S, Trinka E, Kraemer G, Chervoneva I, Werhahn KJ. Impact of carbamazepine, lamotrigine, and levetiracetam on vascular risk markers and lipid‐lowering agents in the elderly. Epilepsia 2018;59(10):1899‐907. - PubMed
Montouris 2007 {published data only}
-
- Montouris G, Alok A, Vuong A, VanLandingham K. An analysis of the efficacy of lamotrigine extended‐release adjunctive therapy in subjects with partial epilepsy by number of concomitant AEDs. Epilepsia 2007;48(Suppl 6):369.
NCT00208520 {published data only}
-
- NCT00208520. Second‐line treatment choice for epilepsy. http://ClinicalTrials.gov/show/NCT00208520 (first received 21 September 2005).
NCT00292461 {published data only}
-
- NCT00292461. A study for evaluating the efficacy and safety of Zonisamide and Lamotrigine (Lamictal) for subjects with refractory simple partial, complex partial or partial with secondary generalized seizures. https://ClinicalTrials.gov/show/NCT00292461 (first received 16 Febraury 2006).
NCT00807989 {published data only}
-
- NCT00807989. The efficacy and safety of low dose combination of LTG and VPA compared to CBZ monotherapy [An open label, randomized, multicenter clinical trial to compare the efficacy and safety of lamotrigine / valproate coadministration and carbamazepine as initial pharmacotherapy in epilepsies (Phase Ⅳ)]. https://ClinicalTrials.gov/show/NCT00807989 (first received 15 December 2008).
NCT01891890 {published data only}
-
- NCT01891890. Cognitive AED outcomes in pediatric localization related epilepsy (COPE). https://ClinicalTrials.gov/show/NCT01891890 (first received 3 July 2013).
NCT02100644 {published data only}
-
- NCT02100644. Valproate dose reduction and its clinical evaluation by introducing Lamotrigine in Japanese women with epilepsy ‐ single arm, multicenter, and open‐label study [Valproate dose reduction and its clinical evaluation by introducing Lamotrigine in Japanese women with epilepsy ‐ single arm, multicenter, and open‐label study]. https://ClinicalTrials.gov/show/NCT02100644 (first received 12 April 2014).
NCT02429596 {published data only}
-
- NCT02429596. A trial of generic substitution of antiepileptic drugs [A randomised controlled trial of generic substitution of antiepileptic drugs]. https://ClinicalTrials.gov/show/NCT02429596 (first received 29 April 2015).
Ohtahara 2008 {published data only}
-
- Ohtahara S, Iinuma K, Fujiwara T, Yamatogi Y. Single‐blind and controlled comparative study of lamotrigine with zonisamide for refractory pediatric epilepsy. Journal of the Japan Epilepsy Society 2008;25(4):425‐40.
Premoli 2017 {published data only}
Privitera 2016 {published data only}
-
- Privitera 2016. Generic‐to‐generic lamotrigine switches in people with epilepsy: the randomised controlled EQUIGEN trial. Lancet Neurology 2016;15(4):365‐72. - PubMed
Sander 1990 {published data only}
-
- Sander JWA, Patsalos PN, Oxley JR, Hamilton MJ, Yuen WC. A randomised double‐blind placebo‐controlled add‐on trial of lamotrigine in patients with severe epilepsy. Epilepsy Research 1990;6:221‐6. - PubMed
Semah 2014 {published data only}
-
- Semah F, Thomas P, Coulbaut S, Derambure P. Early add‐on treatment vs alternative monotherapy in patients with partial epilepsy. Epileptic Disorders 2014;16(2):165‐74. - PubMed
Sethi 2002 {published data only}
-
- Sethi A, Chandra D, Puri V, Mallika V. Gabapentin and lamotrigine in Indian patients of partial epilepsy refractory to carbamazepine. Neurology India 2002;50(3):359‐63. - PubMed
Shinnar 2015 {published data only}
Stolarek 1994 {published data only}
Thangaratinam 2018 {published data only}
-
- Thangaratinam S, Marlin N, Newton S, Weckesser A, Bagary M, Greenhill L. Antiepileptic drug monitoring in pregnancy (EMPiRE): a double‐blind randomised trial on effectiveness and acceptability of monitoring strategies. Health Technology Assessment (Winchester, England) 2018;22(23):1‐152. - PMC - PubMed
Ting 2015 {published data only}
-
- Ting T, Jiang W, Lionberger R, Wong J, Jones JW, Kane M. Generic lamotrigine versus brand‐nameLamictal bioequivalence in patients with epilepsy: a field test of the FDA bioequivalence standard. Epilepsia 2015;56(9):1415‐24. - PubMed
Tomson 2012 {published data only}
-
- Tomson T, Hirsch L, Friedman D, Bester N, Hammer A, Irizarry M, et al. Evaluation of sudden unexpected death in epilepsy (SUDEP) occurring in lamotrigine (LTG) clinical trials. Epilepsia 2012;53(Suppl 5):118. - PubMed
Tomson 2013 {published data only}
-
- Tomson T, Hirsch LJ, Friedman D, Bester N, Hammer A, Irizarry M, et al. Sudden unexpected death in epilepsy in lamotrigine randomized‐controlled trials. Epilepsia 2013;54(1):135‐40. - PubMed
Veendrick‐Meekes 2000 {published data only}
-
- Veendrick‐Meekes MJBM, Beun AM, Carpay JA, Arends LR, Schlosser A. Use of lamotrigine as adjunctive therapy in patients with mental retardation and epilepsy; an interim analysis of a double blind study with evaluation of behavioural effects. Epilepsia 2000;41(Suppl Florence):97.
Wu 2018 {published data only}
-
- Wu D, Chen L, Ji F, Si Y, Sun H. The effects of oxcarbazepine, levetiracetam, and lamotrigine on semen quality, sexual function, and sex hormones in male adults with epilepsy. Epilepsia 2018;59(7):1344‐50. - PubMed
Yamamoto 2012 {published data only}
-
- Yamamoto Y, Inoue Y, Matsuda K, Takahashi Y, Kagawa Y. Influence of concomitant antiepileptic drugs on plasma lamotrigine concentration in adult Japanese epilepsy patients. Biological & Pharmaceutical Bulletin 2012;35(4):487‐93. - PubMed
References to ongoing studies
NCT03689114 {published data only}
-
- NCT03689114. Low vs. standard daily doses of antiepileptic drugs in newly diagnosed, previously untreated epilepsy (STANDLOW) [Efficacy and tolerability of low vs. standard daily doses of antiepileptic drugs in newly diagnosed, previously untreated epilepsy (STANDLOW). A multicenter, randomized, single‐blind, parallel‐group trial]. https://ClinicalTrials.gov/show/NCT03689114 (first received 28 September 2018).
Additional references
Banks 1991
-
- Banks GK, Beran RG. Neuropsychological assessment in lamotrigine treated epileptic patients. Clinical and Experimental Neurology 1991;28:230‐7. - PubMed
Barker‐Haliski 2014
-
- Barker‐Haliski M, Sills GJ, White HS. What are the arguments for and against rational therapy for epilepsy?. Advances in Experimental Medicine & Biology 2014;813:295‐308. - PubMed
Cockerell 1995
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the national general practice study of epilepsy. Lancet 1995;346:140‐4. - PubMed
Commission 1989
-
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30(4):389‐99. - PubMed
Fisher 2005
-
- Fisher RS, Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League A Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46(4):470‐2. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster University. GRADEpro. Hamilton, Canada: McMaster University, 2014.
Hauser 1993
-
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935 ‐1984. Epilepsia 1993;34(3):453‐68. - PubMed
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. - PMC - PubMed
Kirkham 2010
Leach 1995
-
- Leach LJ, Lees G, Riddall DR. Lamotrigine. In: Levy RH, Mattson RH, Medlrum BS editor(s). Mechanisms of Action in Antiepileptic Drugs. 4th Edition. New York: Raven Press, 1995.
Lefebvre 2019
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Loscher 2002
-
- Loscher W. Current status and future directions in the pharmacotherapy of epilepsy. Trends in Pharmacological Sciences 2002;23(3):113‐8. - PubMed
Lyer 2014
-
- Lyer A, Marson A. Pharmacotherapy of focal epilepsy. Expert Opinion on Pharmacotherapy 2014;15(11):1543‐51. - PubMed
Moore 2012
-
- Moore JL, Aggarwal P. Lamotrigine use in pregnancy. Expert Opinion on Pharmacotherapy 2012;13(8):1213‐6. - PubMed
Mula 2013
-
- Mula M. Emerging drugs for focal epilepsy. Expert Opinion on Emerging Drugs 2013;18(1):87‐95. - PubMed
Panebianco 2015
Panebianco 2018
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scheffer 2017
Vajda 2013
-
- Vajda FJE, Dodd S, Horgan D. Lamotrigine in epilepsy, pregnancy and psychiatry ‐ a drug for all seasons?. Journal of Clinical Neuroscience 2013;20(1):13‐6. - PubMed
References to other published versions of this review
Ramaratnam 2001
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

