Damaging de novo missense variants in EEF1A2 lead to a developmental and degenerative epileptic-dyskinetic encephalopathy
- PMID: 32196822
- PMCID: PMC7292794
- DOI: 10.1002/humu.24015
Damaging de novo missense variants in EEF1A2 lead to a developmental and degenerative epileptic-dyskinetic encephalopathy
Abstract
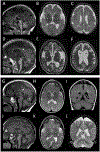
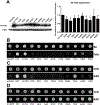
Heterozygous de novo variants in the eukaryotic elongation factor EEF1A2 have previously been described in association with intellectual disability and epilepsy but never functionally validated. Here we report 14 new individuals with heterozygous EEF1A2 variants. We functionally validate multiple variants as protein-damaging using heterologous expression and complementation analysis. Our findings allow us to confirm multiple variants as pathogenic and broaden the phenotypic spectrum to include dystonia/choreoathetosis, and in some cases a degenerative course with cerebral and cerebellar atrophy. Pathogenic variants appear to act via a haploinsufficiency mechanism, disrupting both the protein synthesis and integrated stress response functions of EEF1A2. Our studies provide evidence that EEF1A2 is highly intolerant to variation and that de novo pathogenic variants lead to an epileptic-dyskinetic encephalopathy with both neurodevelopmental and neurodegenerative features. Developmental features may be driven by impaired synaptic protein synthesis during early brain development while progressive symptoms may be linked to an impaired ability to handle cytotoxic stressors.
Keywords: EEF1A2; de novo; dyskinesia; epilepsy; yeast complementation assay.
© 2020 Wiley Periodicals, Inc.
Figures

References
-
- Ann DK, Lin HH, Lee S, Tu ZJ, & Wang E (1992). Characterization of the statin-like S1 and rat elongation factor 1 alpha as two distinctly expressed messages in rat. J Biol Chem, 267(2), 699–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

