New Nocobactin Derivatives with Antimuscarinic Activity, Terpenibactins A-C, Revealed by Genome Mining of Nocardia terpenica IFM 0406
- PMID: 32196864
- PMCID: PMC7497119
- DOI: 10.1002/cbic.202000062
New Nocobactin Derivatives with Antimuscarinic Activity, Terpenibactins A-C, Revealed by Genome Mining of Nocardia terpenica IFM 0406
Abstract
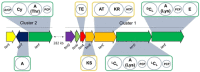
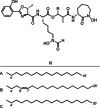
We report a genomics-guided exploration of the metabolic potential of the brasilicardin producer strain Nocardia terpenica IFM 0406. Bioinformatics analysis of the whole genome sequence revealed the presence of a biosynthetic gene cluster presumably responsible for the generation of formerly unknown nocobactin derivatives. Mass spectrometry-assisted isolation led to the identification of three new siderophores, terpenibactins A (1), B (2) and C (3), which belong to the class of nocobactins. Their structures were elucidated by employing spectroscopic techniques. Compounds 1-3 demonstrated inhibitory activity towards the muscarinic M3 receptor, while exhibiting only a low cytotoxicity.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

