The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKα2
- PMID: 32196931
- PMCID: PMC7383816
- DOI: 10.1111/febs.15301
The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKα2
Abstract
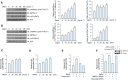
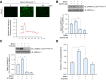
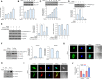
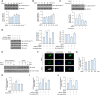
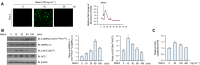
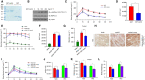
Meteorin-like (metrnl) is a recently identified adipomyokine that beneficially affects glucose metabolism; however, its underlying mechanism of action is not completely understood. We here show that the level of metrnl increases in vitro under electrical pulse stimulation and in vivo in exercised mice, suggesting that metrnl is secreted during muscle contractions. In addition, metrnl increases glucose uptake via the calcium-dependent AMPKα2 pathway in skeletal muscle cells and increases the phosphorylation of HDAC5, a transcriptional repressor of GLUT4, in an AMPKα2-dependent manner. Phosphorylated HDAC5 interacts with 14-3-3 proteins and sequesters them in the cytoplasm, resulting in the activation of GLUT4 transcription. An intraperitoneal injection of recombinant metrnl improved glucose tolerance in mice with high-fat-diet-induced obesity or type 2 diabetes, but not in AMPK β1β2 muscle-specific null mice. Metrnl improves glucose metabolism via AMPKα2 and is a promising therapeutic candidate for glucose-related diseases such as type 2 diabetes.
Keywords: AMPK; Metrnl; adipomyokine; glucose uptake; type 2 diabetes.
© 2020 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


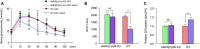
References
-
- Pedersen BK (2011) Muscles and their myokines. J Exp Biol 214, 337–346. - PubMed
-
- Barra NG, Chew MV, Holloway AC & Ashkar AA (2012) Interleukin‐15 treatment improves glucose homeostasis and insulin sensitivity in obese mice. Diabetes Obes Metab 14, 190–193. - PubMed
-
- Alvarez B, Carbó N, López‐Soriano J, Drivdahl RH, Busquets S, López‐Soriano FJ, Argilés JM & Quinn LS (2002) Effects of interleukin‐15 (IL‐15) on adipose tissue mass in rodent obesity models: evidence for direct IL‐15 action on adipose tissue. Biochim Biophys Acta 1570, 33–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

