Localization of Macrophages in the Human Lung via Design-based Stereology
- PMID: 32197050
- PMCID: PMC7233346
- DOI: 10.1164/rccm.201911-2105OC
Localization of Macrophages in the Human Lung via Design-based Stereology
Abstract
Rationale: Interstitial macrophages (IMs) and airspace macrophages (AMs) play critical roles in lung homeostasis and host defense, and are central to the pathogenesis of a number of lung diseases. However, the absolute numbers of macrophages and the precise anatomic locations they occupy in the healthy human lung have not been quantified.Objectives: To determine the precise number and anatomic location of human pulmonary macrophages in nondiseased lungs and to quantify how this is altered in chronic cigarette smokers.Methods: Whole right upper lobes from 12 human donors without pulmonary disease (6 smokers and 6 nonsmokers) were evaluated using design-based stereology. CD206 (cluster of differentiation 206)-positive/CD43+ AMs and CD206+/CD43- IMs were counted in five distinct anatomical locations using the optical disector probe.Measurements and Main Results: An average of 2.1 × 109 IMs and 1.4 × 109 AMs were estimated per right upper lobe. Of the AMs, 95% were contained in diffusing airspaces and 5% in airways. Of the IMs, 78% were located within the alveolar septa, 14% around small vessels, and 7% around the airways. The local density of IMs was greater in the alveolar septa than in the connective tissue surrounding the airways or vessels. The total number and density of IMs was 36% to 56% greater in the lungs of cigarette smokers versus nonsmokers.Conclusions: The precise locations occupied by pulmonary macrophages were defined in nondiseased human lungs from smokers and nonsmokers. IM density was greatest in the alveolar septa. Lungs from chronic smokers had increased IM numbers and overall density, supporting a role for IMs in smoking-related disease.
Keywords: alveolar; cigarette smokers; emphysema; interstitial; morphometry.
Figures
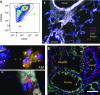
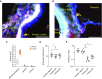
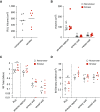
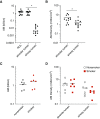
Comment in
-
Know Where You Are: Pulmonary Macrophage Locations in the Human Lung.Am J Respir Crit Care Med. 2020 May 15;201(10):1169-1170. doi: 10.1164/rccm.202002-0235ED. Am J Respir Crit Care Med. 2020. PMID: 32228390 Free PMC article. No abstract available.
References
-
- Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18–25. - PubMed
-
- Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C, et al. Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity. 2017;46:457–473. - PubMed
-
- Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363:eaau0964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

