Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II
- PMID: 32197113
- PMCID: PMC7160308
- DOI: 10.1016/j.stemcr.2020.02.006
Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II
Abstract
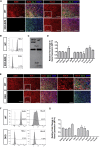
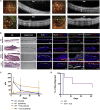
Human embryonic stem cell-derived retinal pigment epithelial (hESC-RPE) cells could serve as a replacement therapy in advanced stages of age-related macular degeneration. However, allogenic hESC-RPE transplants trigger immune rejection, supporting a strategy to evade their immune recognition. We established single-knockout beta-2 microglobulin (SKO-B2M), class II major histocompatibility complex transactivator (SKO-CIITA) and double-knockout (DKO) hESC lines that were further differentiated into corresponding hESC-RPE lines lacking either surface human leukocyte antigen class I (HLA-I) or HLA-II, or both. Activation of CD4+ and CD8+ T-cells was markedly lower by hESC-RPE DKO cells, while natural killer cell cytotoxic response was not increased. After transplantation of SKO-B2M, SKO-CIITA, or DKO hESC-RPEs in a preclinical rabbit model, donor cell rejection was reduced and delayed. In conclusion, we have developed cell lines that lack both HLA-I and -II antigens, which evoke reduced T-cell responses in vitro together with reduced rejection in a large-eyed animal model.
Keywords: HLA-I knockout; HLA-II knockout; cellular therapy; human embryonic stem cells; immune evasion; retinal pigment epithelium; subretinal injection; transplantation rejection; xenogeneic transplant; xenograft model.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Bradley J.A., Bolton E.M., Pedersen R.A. Stem cell medicine encounters the immune system. Nat. Rev. Immunol. 2002;2:859–871. - PubMed
-
- da Cruz L., Fynes K., Georgiadis O., Kerby J., Luo Y.H., Ahmado A., Vernon A., Daniels J.T., Nommiste B., Hasan S.M. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018;36:328–337. - PubMed
-
- de Rham C., Villard J. How to cross immunogenetic hurdles to human embryonic stem cell transplantation. Semin. Immunopathol. 2011;33:525–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

