Administration of Bacterial Lipopolysaccharide during Early Postnatal Ontogenesis Induces Transient Impairment of Long-Term Synaptic Plasticity Associated with Behavioral Abnormalities in Young Rats
- PMID: 32197321
- PMCID: PMC7151710
- DOI: 10.3390/ph13030048
Administration of Bacterial Lipopolysaccharide during Early Postnatal Ontogenesis Induces Transient Impairment of Long-Term Synaptic Plasticity Associated with Behavioral Abnormalities in Young Rats
Abstract
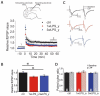
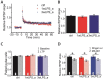
Infectious diseases in early postnatal ontogenesis often result in cognitive impairments, particularly learning and memory. The essential foundation of learning and memory is long-term synaptic plasticity, which depends on N-methyl-D-aspartate (NMDA) receptors. In the present study, bacterial infection was modeled by treating rat pups with bacterial lipopolysaccharide (LPS, 25 µg/kg) three times, during either the first or the third week of life. These time points are critical for the maturation of NMDA receptors. We assessed the effects of LPS treatments on the properties of long-term potentiation (LTP) in the CA1 hippocampus of young (21-23 days) and adolescent (51-55 days) rats. LTP magnitude was found to be significantly reduced in both groups of young rats, which also exhibited investigative and motor behavior disturbances in the open field test. No changes were observed in the main characteristics of synaptic transmission, although the LTP induction mechanism was disturbed. In rats treated with LPS during the third week, the NMDA-dependent form of LTP was completely suppressed, and LTP switched to the Type 1 metabotropic glutamate receptor (mGluR1)-dependent form. These impairments of synaptic plasticity and behavior were temporary. In adolescent rats, no difference was observed in LTP properties between the control and experimental groups. Lastly, the investigative and motor behavior parameters in both groups of adult rats were similar.
Keywords: bacterial lipopolysaccharide; early life; hippocampus; long-term potentiation; open field test.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





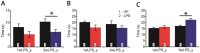
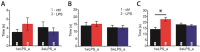
Similar articles
-
Exposure to bacterial lipopolysaccharidein early life affects the expression of ionotropic glutamate receptor genes and is accompanied by disturbances in long-term potentiation and cognitive functions in young rats.Brain Behav Immun. 2020 Nov;90:3-15. doi: 10.1016/j.bbi.2020.07.034. Epub 2020 Jul 26. Brain Behav Immun. 2020. PMID: 32726683
-
Disturbance of Metabotropic Glutamate Receptor-Mediated Long-Term Depression (mGlu-LTD) of Excitatory Synaptic Transmission in the Rat Hippocampus After Prenatal Immune Challenge.Neurochem Res. 2019 Mar;44(3):609-616. doi: 10.1007/s11064-018-2476-0. Epub 2018 Jan 20. Neurochem Res. 2019. PMID: 29353373
-
Characterization of the anoxia-induced long-term synaptic potentiation in area CA1 of the rat hippocampus.Br J Pharmacol. 1997 Oct;122(4):671-81. doi: 10.1038/sj.bjp.0701409. Br J Pharmacol. 1997. PMID: 9375963 Free PMC article.
-
Glutamatergic components underlying lead-induced impairments in hippocampal synaptic plasticity.Neurotoxicology. 2000 Dec;21(6):1057-68. Neurotoxicology. 2000. PMID: 11233752 Review.
-
Plasticity, hippocampal place cells, and cognitive maps.Arch Neurol. 2001 Jun;58(6):874-81. doi: 10.1001/archneur.58.6.874. Arch Neurol. 2001. PMID: 11405801 Review.
Cited by
-
Roxadustat (FG-4592) abated lipopolysaccharides-induced depressive-like symptoms via PI3K signaling.Front Mol Neurosci. 2023 Mar 15;16:1048985. doi: 10.3389/fnmol.2023.1048985. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37008780 Free PMC article.
-
Animal Models of Febrile Seizures: Limitations and Recent Advances in the Field.Cells. 2024 Nov 16;13(22):1895. doi: 10.3390/cells13221895. Cells. 2024. PMID: 39594643 Free PMC article. Review.
-
Intermittent systemic exposure to lipopolysaccharide-induced inflammation disrupts hippocampal long-term potentiation and impairs cognition in aging male mice.Brain Behav Immun. 2023 Feb;108:279-291. doi: 10.1016/j.bbi.2022.12.013. Epub 2022 Dec 19. Brain Behav Immun. 2023. PMID: 36549577 Free PMC article.
References
-
- Golia M.T., Poggini S., Alboni S., Garofalo S., Albanese N.C., Viglione A., Ajmone-Cat M.A., St-Pierre A., Brunello N., Limatola C., et al. Interplay between inflammation and neural plasticity: Both immune activation and suppression impair LTP and BDNF expression. Brain Behav. Immun. 2019;81:484–494. doi: 10.1016/j.bbi.2019.07.003. - DOI - PubMed
-
- Kubera M., Curzytek K., Duda W., Leskiewicz M., Basta-Kaim A., Budziszewska B., Roman A., Zajicova A., Holan V., Szczesny E., et al. A new animal model of (chronic) depression induced by repeated and intermittent lipopolysaccharide administration for 4 months. Brain Behav. Immun. 2013;31:96–104. doi: 10.1016/j.bbi.2013.01.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

