Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors
- PMID: 32197322
- PMCID: PMC7175173
- DOI: 10.3390/biom10030464
Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors
Abstract
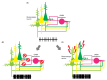
It is widely accepted that glutamate-mediated neuronal hyperexcitation plays a causative role in eliciting seizures. Among glutamate receptors, the roles of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors in physiological and pathological conditions represent major clinical research targets. It is well known that agonists of NMDA or AMPA receptors can elicit seizures in animal or human subjects, while antagonists have been shown to inhibit seizures in animal models, suggesting a potential role for NMDA and AMPA receptor antagonists in anti-seizure drug development. Several such drugs have been evaluated in clinical studies; however, the majority, mainly NMDA-receptor antagonists, failed to demonstrate adequate efficacy and safety for therapeutic use, and only an AMPA-receptor antagonist, perampanel, has been approved for the treatment of some forms of epilepsy. These results suggest that a misunderstanding of the role of each glutamate receptor in the ictogenic process may underlie the failure of these drugs to demonstrate clinical efficacy and safety. Accumulating knowledge of both NMDA and AMPA receptors, including pathological gene mutations, roles in autoimmune epilepsy, and evidence from drug-discovery research and pharmacological studies, may provide valuable information enabling the roles of both receptors in ictogenesis to be reconsidered. This review aimed to integrate information from several studies in order to further elucidate the specific roles of NMDA and AMPA receptors in epilepsy.
Keywords: AMPA; NMDA; epilepsy; glutamate; ictogenesis; pharmacology.
Conflict of interest statement
Takahisa Hanada is an employee of Eisai Co., Ltd.
Figures

References
-
- Cervetto C., Frattaroli D., Venturini A., Passalacqua M., Nobile M., Alloisio S., Tacchetti C., Maura G., Agnati L.F., Marcoli M. Calcium-permeable AMPA receptors trigger vesicular glutamate release from Bergmann gliosomes. Neuropharmacology. 2015;99:396–407. doi: 10.1016/j.neuropharm.2015.08.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

