Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms
- PMID: 32197363
- PMCID: PMC7139924
- DOI: 10.3390/ijms21062080
Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms
Abstract
Venous thromboembolism (VTE) is a pathology encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE) associated with high morbidity and mortality. Because patients often present after a thrombus has already formed, the mechanisms that drive DVT resolution are being investigated in search of treatment. Herein, we review the current literature, including the molecular mechanisms of fibrinolysis and collagenolysis, as well as the critical cellular roles of macrophages, neutrophils, and endothelial cells. We propose two general models for the operation of the immune system in the context of venous thrombosis. In early thrombus resolution, neutrophil influx stabilizes the tissue through NETosis. Meanwhile, macrophages and intact neutrophils recognize the extracellular DNA by the TLR9 receptor and induce fibrosis, a complimentary stabilization method. At later stages of resolution, pro-inflammatory macrophages police the thrombus for pathogens, a role supported by both T-cells and mast cells. Once they verify sterility, these macrophages transform into their pro-resolving phenotype. Endothelial cells both coat the stabilized thrombus, a necessary early step, and can undergo an endothelial-mesenchymal transition, which impedes DVT resolution. Several of these interactions hold promise for future therapy.
Keywords: NETosis; collagenolysis; deep vein thrombosis; endothelial cell; fibrinolysis; immune system; macrophage; neutrophil; stability; venous thromboembolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
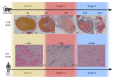

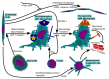
References
-
- Kochanek K.D., Murphy S.L., Xu J., Arias E. National Vital Statistics Reports Volume 68, Number 9, 24 June 2019. Deaths: Final Data for 2017. [(accessed on 12 September 2019)]; Available online: https://www.cdc.gov/nchs/products/index.htm. - PubMed
-
- Delluc A., Tromeur C., le Ven F., Gouillou M., Paleiron N., Bressollette L., Nonent M., Salaun P.-Y., Lacut K., Leroyer C., et al. Current incidence of venous thromboembolism and comparison with 1998: A community-based study in Western France. Thromb. Haemost. 2016;116:967–974. doi: 10.1160/TH16-03-0205. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

