Opioid Pharmacology under the Microscope
- PMID: 32198210
- PMCID: PMC7562971
- DOI: 10.1124/mol.119.119321
Opioid Pharmacology under the Microscope
Abstract
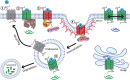
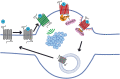
The powerful analgesic effects of opioid drugs have captivated the interest of physicians and scientists for millennia, and the ability of opioid drugs to produce serious undesired effects has been recognized for a similar period of time (Kieffer and Evans, 2009). Many of these develop progressively with prolonged or repeated drug use and then persist, motivating particular interest in understanding how opioid drugs initiate adaptive or maladaptive modifications in neural function or regulation. Exciting advances have been made over the past several years in elucidating drug-induced changes at molecular, cellular, and physiologic scales of analysis. The present review will highlight some recent cellular studies that we believe bridge across scales and will focus on optical imaging approaches that put opioid drug action "under the microscope." SIGNIFICANCE STATEMENT: Opioid receptors are major pharmacological targets, but their signaling at the cellular level results from a complex interplay between pharmacology, regulation, subcellular localization, and membrane trafficking. This minireview discusses recent advances in understanding the cellular biology of opioid receptors, emphasizing particular topics discussed at the 50th anniversary of the International Narcotics Research Conference. Our goal is to highlight distinct signaling and regulatory properties emerging from the cellular biology of opioid receptors and discuss potential relevance to therapeutics.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

