Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis
- PMID: 32198218
- PMCID: PMC9488897
- DOI: 10.1183/16000617.0146-2019
Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis
Abstract
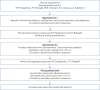
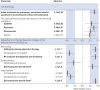
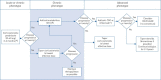
Pulmonary sarcoidosis presents substantial management challenges, with limited evidence on effective therapies and phenotypes. In the absence of definitive evidence, expert consensus can supply clinically useful guidance in medicine. An international panel of 26 experts participated in a Delphi process to identify consensus on pharmacological management in sarcoidosis with the development of preliminary recommendations.The modified Delphi process used three rounds. The first round focused on qualitative data collection with open-ended questions to ensure comprehensive inclusion of expert concepts. Rounds 2 and 3 applied quantitative assessments using an 11-point Likert scale to identify consensus.Key consensus points included glucocorticoids as initial therapy for most patients, with non-biologics (immunomodulators), usually methotrexate, considered in severe or extrapulmonary disease requiring prolonged treatment, or as a steroid-sparing intervention in cases with high risk of steroid toxicity. Biologic therapies might be considered as additive therapy if non-biologics are insufficiently effective or are not tolerated with initial biologic therapy, usually with a tumour necrosis factor-α inhibitor, typically infliximab.The Delphi methodology provided a platform to gain potentially valuable insight and interim guidance while awaiting evidenced-based contributions.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: F.F. Rahaghi reports grants and consulting fees from Mallinckrodt, during the conduct of the study. Conflict of interest: R.P. Baughman reports grants and personal fees from Malllinckrodt, Novartis and Celgene, grants from Gilead, Genentech, Bayer and West Pharmaceutical, during the conduct of the study. Conflict of interest: L.A. Saketkoo has nothing to disclose. Conflict of interest: N.J. Sweiss has nothing to disclose. Conflict of interest: J.B. Barney has nothing to disclose. Conflict of interest: S.S. Birring has nothing to disclose. Conflict of interest: U. Costabel has nothing to disclose. Conflict of interest: E.D. Crouser has no relevant conflicts of interest to disclose. Conflict of interest: M. Drent has nothing to disclose. Conflict of interest: A.K. Gerke has nothing to disclose. Conflict of interest: J.C. Grutters has nothing to disclose. Conflict of interest: N.Y. Hamzeh has a patent pending from Prothena Inc. Conflict of interest: I. Huizar has no relevant conflicts of interest to disclose. Conflict of interest: W.E. James has nothing to disclose. Conflict of interest: S. Kalra has nothing to disclose. Conflict of interest: S. Kullberg has nothing to disclose. Conflict of interest: H. Li has nothing to disclose. Conflict of interest: E.E. Lower has nothing to disclose. Conflict of interest: L.A. Maier reports grants from National Institutes of Health: 1R01 HL127461-01A, 1R01HL11487-01A1, R01HL136681-01A1, 1R21 128738A-02, R21 HL140012-1, outside the submitted work. Conflict of interest: M. Mirsaeidi reports grants and personal fees from Mallinckrodt, outside the submitted work. Conflict of interest: J. Müller-Quernheim has nothing to disclose. Conflict of interest: E.M. Carmona Porquera reports other from ReSaph (Gilead), personal fees from American College of Chest Physicians (CHEST), outside the submitted work. Conflict of interest: L. Samavati participated in the Questcor Advisory Board Meeting 2014 and received $6500 compensation. Conflict of interest: D. Valeyre reports personal fees from Roche, Boehringer Ingelheim, AstraZeneca and Boehringer Ingelheim, outside the submitted work. Conflict of interest: M.B. Scholand reports other from Boehringer Ingelheim, Fibrogen, Global Blood Therapeutics and Genetech, outside the submitted work. In addition, M.B. Scholand has a patent Apparatus, Compositions and Methods for Assessment of Chronic Obstructive Pulmonary Disease Progression among Rapid and Slow Decline Conditions issued.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
