Impact of a short version of the CONSORT checklist for peer reviewers to improve the reporting of randomised controlled trials published in biomedical journals: study protocol for a randomised controlled trial
- PMID: 32198306
- PMCID: PMC7103787
- DOI: 10.1136/bmjopen-2019-035114
Impact of a short version of the CONSORT checklist for peer reviewers to improve the reporting of randomised controlled trials published in biomedical journals: study protocol for a randomised controlled trial
Abstract
Introduction: Transparent and accurate reporting is essential for readers to adequately interpret the results of a study. Journals can play a vital role in improving the reporting of published randomised controlled trials (RCTs). We describe an RCT to evaluate our hypothesis that asking peer reviewers to check whether the most important and poorly reported CONsolidated Standards of Reporting Trials (CONSORT) items are adequately reported will result in higher adherence to CONSORT guidelines in published RCTs.
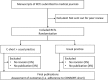
Methods and analysis: Manuscripts presenting the primary results of RCTs submitted to participating journals will be randomised to either the intervention group (peer reviewers will receive a reminder and short explanation of the 10 most important and poorly reported CONSORT items; they will be asked to check if these items are reported in the submitted manuscript) or a control group (usual journal practice). The primary outcome will be the mean proportion of the 10 items that are adequately reported in the published articles. Peer reviewers and manuscript authors will not be informed of the study hypothesis, design or intervention. Outcomes will be assessed in duplicate from published articles by two data extractors (at least one blinded to the intervention). We will enrol eligible manuscripts until a minimum of 83 articles per group (166 in total) are published.
Ethics and dissemination: This pragmatic RCT was approved by the Medical Sciences Interdivisional Research Ethics Committee of the University of Oxford (R62779/RE001). If this intervention is effective, it could be implemented by all medical journals without requiring large additional resources at journal level. Findings will be disseminated through presentations in relevant conferences and peer-reviewed publications. This trial is registered on the Open Science Framework (https://osf.io/c4hn8).
Keywords: medical education & training; protocols & guidelines; quality in healthcare; statistics & research methods.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests, and roles of the steering committee: SS is employed by the British Medical Journal (BMJ). IP and AC are employed by the Public Library of Science. DM, SH and IB are members of the CONsolidated Standards for Reporting Trials (CONSORT) executive and authors of the CONSORT 2010 statement. DM and PR are members of the EQUATOR (Enhancing the Quality and Transparency of Research) network steering group. MMS is a meta-researcher and reporting guideline developer, enthusiast and disseminator; he may therefore overestimate the importance of this project. All authors have declared that no other competing interests exist: the principal investigator (BS) is responsible for the preparation and the revisions of the study protocol, organising meetings of the steering committee, recruiting and randomising eligible manuscripts as well as the publication of study reports. The steering committee (IB, MB, SH, DM, PR, BS, MMS and SS) is responsible for revising the protocol, defining and validating the additional short explanation for each CONSORT item, advising on study implementation and for publishing the results of this study. MMS is responsible for the sample size calculation and the statistical analyses.
Figures
Similar articles
-
Reminding Peer Reviewers of Reporting Guideline Items to Improve Completeness in Published Articles: Primary Results of 2 Randomized Trials.JAMA Netw Open. 2023 Jun 1;6(6):e2317651. doi: 10.1001/jamanetworkopen.2023.17651. JAMA Netw Open. 2023. PMID: 37294569 Free PMC article.
-
A protocol of a cross-sectional study evaluating an online tool for early career peer reviewers assessing reports of randomised controlled trials.BMJ Open. 2017 Sep 15;7(9):e017462. doi: 10.1136/bmjopen-2017-017462. BMJ Open. 2017. PMID: 28918414 Free PMC article.
-
Reporting Quality of Randomized Controlled Trials of Periodontal Diseases in Journal Abstracts-A Cross-sectional Survey and Bibliometric Analysis.J Evid Based Dent Pract. 2018 Jun;18(2):130-141.e22. doi: 10.1016/j.jebdp.2017.08.005. Epub 2017 Sep 21. J Evid Based Dent Pract. 2018. PMID: 29747793
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Reporting randomised trials of social and psychological interventions: the CONSORT-SPI 2018 Extension.Trials. 2018 Jul 31;19(1):407. doi: 10.1186/s13063-018-2733-1. Trials. 2018. PMID: 30060754 Free PMC article.
Cited by
-
Development of a checklist to detect errors in meta-analyses in systematic reviews of interventions: study protocol.F1000Res. 2021 Jun 8;10:455. doi: 10.12688/f1000research.53034.1. eCollection 2021. F1000Res. 2021. PMID: 34249342 Free PMC article.
-
Did an introduction of CONSORT for abstracts guidelines improve reporting quality of randomised controlled trials' abstracts on Helicobacter pylori infection? Observational study.BMJ Open. 2022 Mar 30;12(3):e054978. doi: 10.1136/bmjopen-2021-054978. BMJ Open. 2022. PMID: 35354625 Free PMC article.
-
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71. BMJ. 2021. PMID: 33782057 Free PMC article.
-
[The PRISMA 2020 statement: an updated guideline for reporting systematic reviewsDeclaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas].Rev Panam Salud Publica. 2022 Dec 30;46:e112. doi: 10.26633/RPSP.2022.112. eCollection 2022. Rev Panam Salud Publica. 2022. PMID: 36601438 Free PMC article. Portuguese.
-
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.Syst Rev. 2021 Mar 29;10(1):89. doi: 10.1186/s13643-021-01626-4. Syst Rev. 2021. PMID: 33781348 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources