Glioblastoma infiltration of both tumor- and virus-antigen specific cytotoxic T cells correlates with experimental virotherapy responses
- PMID: 32198420
- PMCID: PMC7083912
- DOI: 10.1038/s41598-020-61736-2
Glioblastoma infiltration of both tumor- and virus-antigen specific cytotoxic T cells correlates with experimental virotherapy responses
Abstract
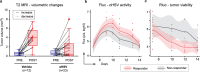
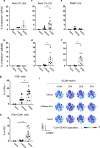
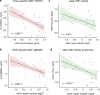
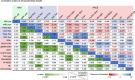
The mode of action for oncolytic viruses (OVs) in cancer treatment is thought to depend on a direct initial cytotoxic effect against infected tumor cells and subsequent activation of immune cell responses directed against the neoplasm. To study both of these effects in a mouse model of glioblastoma (GBM), we employed murine GBM cells engineered to constitutively express the type I Herpes Simplex Virus (HSV1) HSV-1 receptor, nectin-1, to allow for more efficient infection and replication by oncolytic HSV (oHSV). These cells were further engineered with a surrogate tumor antigen to facilitate assays of T cell activity. We utilized MRI-based volumetrics to measure GBM responses after injection with the oHSV and bioluminescent imaging (BLI) to determine oHSV replicative kinetics in the injected tumor mass. We found increased infiltration of both surrogate tumor antigen- and oHSV antigen-specific CD8+ T cells within 7 days after oHSV injection. There was no increase in tumor infiltrating CD8+ T cells expressing "exhaustion" markers, yet oHSV infection led to a reduction in PD-1+ CD8+ T cells in injected GBMs and an increase in IFNγ+ CD8+ T cells. There was a significant direct correlation between oHSV-mediated reduction in GBM volume and increased infiltration of both viral and tumor antigen-specific CD8+ T cells, as well as oHSV intratumoral gene activity. These findings imply that CD8+ T cell cytotoxicity against both tumor and viral antigens as well as intratumoral oHSV gene expression are important in oHSV-mediated GBM therapy.
Conflict of interest statement
E.A.C. is currently a paid advisor to Advantagene Inc., Insightec, Inc., and DNAtrix Inc. and has equity interest in DNAtrix; He has received research support from Advantagene, NewLink Genetics and Amgen. H.N. has received research support from NewLink Genetics. H.N. was a technical consultant for Oncorus INC and has received compensation. W.F.G is a consultant for Oncorus INC and has received compensation. D.A.R. (DFCI) has received research support from Acerta Phamaceuticals; Agenus; Celldex; EMD Serono; Incyte; Inovio; Midatech; Omniox; Tragara. D.A.R. is currently paid advisory/consultant for Abbvie; Advantagene; Agenus; Amgen; Bayer; Bristol-Myers Squibb; Celldex; DelMar; EMD Serono; Genentech/Roche; Inovio; Merck; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Taiho Oncology, Inc. Other authors declare no competing interests. E.A.C. has also advised Oncorus, Merck, Tocagen, Ziopharm, Stemgen, NanoTx., Ziopharm Oncology, Cerebral Therapeutics, Genenta. Merck, Janssen, Karcinolysis, Shanaghai Biotech. H.N. and E.A.C are named inventors on patents related to oncolytic HSV1.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

