Polycyclic aromatic chains on metals and insulating layers by repetitive [3+2] cycloadditions
- PMID: 32198456
- PMCID: PMC7083871
- DOI: 10.1038/s41467-020-15210-2
Polycyclic aromatic chains on metals and insulating layers by repetitive [3+2] cycloadditions
Erratum in
-
Author Correction: Polycyclic aromatic chains on metals and insulating layers by repetitive [3+2] cycloadditions.Nat Commun. 2020 Aug 4;11(1):3937. doi: 10.1038/s41467-020-17857-3. Nat Commun. 2020. PMID: 32753628 Free PMC article.
Abstract
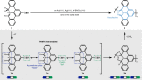
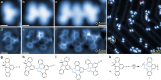
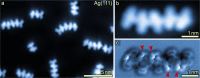
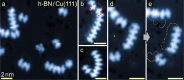
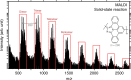
The vast potential of organic materials for electronic, optoelectronic and spintronic devices entails substantial interest in the fabrication of π-conjugated systems with tailored functionality directly at insulating interfaces. On-surface fabrication of such materials on non-metal surfaces remains to be demonstrated with high yield and selectivity. Here we present the synthesis of polyaromatic chains on metallic substrates, insulating layers, and in the solid state. Scanning probe microscopy shows the formation of azaullazine repeating units on Au(111), Ag(111), and h-BN/Cu(111), stemming from intermolecular homo-coupling via cycloaddition reactions of CN-substituted polycyclic aromatic azomethine ylide (PAMY) intermediates followed by subsequent dehydrogenation. Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry demonstrates that the reaction also takes place in the solid state in the absence of any catalyst. Such intermolecular cycloaddition reactions are promising methods for direct synthesis of regioregular polyaromatic polymers on arbitrary insulating surfaces.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Dutta S, Pati SK. Novel properties of graphene nanoribbons: a review. J. Mater. Chem. 2010;20:8207. doi: 10.1039/c0jm00261e. - DOI
-
- Yagmurcukardes M, Peeters FM, Senger RT, Sahin H. Nanoribbons: From fundamentals to state-of-the-art applications. Appl. Phys. Rev. 2016;3:041302. doi: 10.1063/1.4966963. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

