Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19)
- PMID: 32198501
- PMCID: PMC7184472
- DOI: 10.1093/cid/ciaa310
Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19)
Abstract
Background: The emergence of coronavirus disease 2019 (COVID-19) is a major healthcare threat. The current method of detection involves a quantitative polymerase chain reaction (qPCR)-based technique, which identifies the viral nucleic acids when present in sufficient quantity. False-negative results can be achieved and failure to quarantine the infected patient would be a major setback in containing the viral transmission. We aim to describe the time kinetics of various antibodies produced against the 2019 novel coronavirus (SARS-CoV-2) and evaluate the potential of antibody testing to diagnose COVID-19.
Methods: The host humoral response against SARS-CoV-2, including IgA, IgM, and IgG response, was examined by using an ELISA-based assay on the recombinant viral nucleocapsid protein. 208 plasma samples were collected from 82 confirmed and 58 probable cases (qPCR negative but with typical manifestation). The diagnostic value of IgM was evaluated in this cohort.
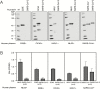
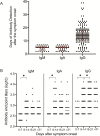
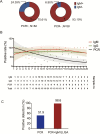
Results: The median duration of IgM and IgA antibody detection was 5 (IQR, 3-6) days, while IgG was detected 14 (IQR, 10-18) days after symptom onset, with a positive rate of 85.4%, 92.7%, and 77.9%, respectively. In confirmed and probable cases, the positive rates of IgM antibodies were 75.6% and 93.1%, respectively. The detection efficiency by IgM ELISA is higher than that of qPCR after 5.5 days of symptom onset. The positive detection rate is significantly increased (98.6%) when combining IgM ELISA assay with PCR for each patient compared with a single qPCR test (51.9%).
Conclusions: The humoral response to SARS-CoV-2 can aid in the diagnosis of COVID-19, including subclinical cases.
Keywords: COVID-19; ELISA; antibody; diagnosis; novel coronavirus.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Reply to Yamaoka, et al.Clin Infect Dis. 2021 Apr 8;72(7):1293. doi: 10.1093/cid/ciaa687. Clin Infect Dis. 2021. PMID: 33830223 No abstract available.
References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) situation report—23 2020.World Health Organization; Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 13 February 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

