CYD Tetravalent Dengue Vaccine Performance by Baseline Immune Profile (Monotypic/Multitypic) in Dengue-Seropositive Individuals
- PMID: 32198515
- PMCID: PMC8130022
- DOI: 10.1093/cid/ciaa304
CYD Tetravalent Dengue Vaccine Performance by Baseline Immune Profile (Monotypic/Multitypic) in Dengue-Seropositive Individuals
Abstract
Background: The immune profile of dengue-experienced individuals is a determinant of dengue reinfection severity risk. Individuals with a single prior dengue infection (monotypic) are at highest risk for severe disease, while individuals with ≥ 2 prior dengue infections (multitypic) are at lower risk. The tetravalent dengue vaccine (CYD-TDV) has shown efficacy in the prevention of dengue in individuals with prior dengue infection. We estimated efficacy in individuals with monotypic or multitypic immune profiles.
Methods: Participants enrolled in the immunogenicity subsets of 2 randomized placebo-controlled phase 3 studies (CYD14, NCT01373281; CYD15, NCT01374516) were classified as either monotypic or multitypic, based on measured baseline dengue plaque reduction neutralization test. Vaccine efficacy (VE) against symptomatic virologically confirmed dengue (VCD) was assessed over 25 months and against VCD hospitalization over 6 years.
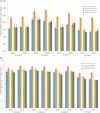
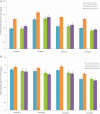
Results: Of 3927 participants in the immunogenicity subsets, 496 and 257 in the CYD-TDV and placebo groups, respectively, were classified as monotypic immune, and 1227 and 612, respectively, as multitypic immune. VE against symptomatic VCD was 77.4% (95% CI, 56.4%-88.2%) for monotypic and 89.2% (95% CI, 71.5%-95.9%) for multitypic profiles, with corresponding absolute risk reductions (ARRs) of 4.48% (95% CI, 2.32%-6.65%) for monotypics and 1.67% (95% CI, .89%-2.46%) for multitypics. VE against hospitalized VCD was 75.3% (95% CI, 42.7%-90.2%) in monotypics and 81.2% (95% CI, 21.7%-96.8%) in multitypics, with ARRs of 0.95% (95% CI, .37%-1.53%) for monotypics and 0.18% (95% CI, .02%-.34%) for multitypics.
Conclusions: CYD-TDV benefits individuals with monotypic and multitypic immune profiles. Larger public health benefit is expected to derive from the protection of individuals with a monotypic immune profile.
Keywords: CYD14; CYD15; dengue; monotypic; multitypic.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Dengue vaccine: WHO position paper, September 2018—recommendations. Vaccine 2019; 37:4848–9. - PubMed
-
- Gibbons RV, Kalanarooj S, Jarman RG, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 2007; 77:910–3. - PubMed
-
- Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018; 379:327–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

