Management of myelofibrosis after ruxolitinib failure
- PMID: 32198525
- PMCID: PMC7237516
- DOI: 10.1007/s00277-020-04002-9
Management of myelofibrosis after ruxolitinib failure
Abstract
Myelofibrosis is a BCR-ABL1-negative myeloproliferative neoplasm characterized by anemia, progressive splenomegaly, extramedullary hematopoiesis, bone marrow fibrosis, constitutional symptoms, leukemic progression, and shortened survival. Constitutive activation of the Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway, and other cellular pathways downstream, leads to myeloproliferation, proinflammatory cytokine expression, and bone marrow remodeling. Transplant is the only curative option for myelofibrosis, but high rates of morbidity and mortality limit eligibility. Several prognostic models have been developed to facilitate treatment decisions. Until the recent approval of fedratinib, a JAK2 inhibitor, ruxolitinib was the only available JAK inhibitor for treatment of intermediate- or high-risk myelofibrosis. Ruxolitinib reduces splenomegaly to some degree in almost all treated patients; however, many patients cannot tolerate ruxolitinib due to dose-dependent drug-related cytopenias, and even patients with a good initial response often develop resistance to ruxolitinib after 2-3 years of therapy. Currently, there is no consensus definition of ruxolitinib failure. Until fedratinib approval, strategies to overcome ruxolitinib resistance or intolerance were mainly different approaches to continued ruxolitinib therapy, including dosing modifications and ruxolitinib rechallenge. Fedratinib and two other JAK2 inhibitors in later stages of clinical development, pacritinib and momelotinib, have been shown to induce clinical responses and improve symptoms in patients previously treated with ruxolitinib. Fedratinib induces robust spleen responses, and pacritinib and momelotinib may have preferential activity in patients with severe cytopenias. Reviewed here are strategies to ameliorate ruxolitinib resistance or intolerance, and outcomes of clinical trials in patients with myelofibrosis receiving second-line JAK inhibitors after ruxolitinib treatment.
Keywords: Fedratinib; Momelotinib; Myelofibrosis; Pacritinib; Ruxolitinib.
Conflict of interest statement
CNH received research fees from Novartis and speaker payments from Novartis, Celgene, Roche, and Jannsen and participated in advisory boards for Sierra Oncology, Promedior, Celgene, and Novartis. RAM received consultant fees from Novartis, Sierra, and La Jolla Pharmaceutical Company and received research support from Celgene, Incyte, Abbvie, Samus Therapeutics, Genotech, Promedior, and CTI Biopharma. NC declares no conflicts of interest.
Figures
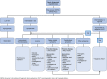
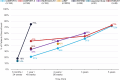
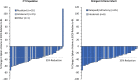
References
-
- Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, Gotlib J, Dupriez B, Pardanani A, Harrison C, Hoffman R, Gisslinger H, Kroger N, Thiele J, Barbui T, Barosi G. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395–1398. doi: 10.1182/blood-2013-03-488098. - DOI - PMC - PubMed
-
- Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, Finke C, Score J, Gangat N, Mannarelli C, Ketterling RP, Rotunno G, Knudson RA, Susini MC, Laborde RR, Spolverini A, Pancrazzi A, Pieri L, Manfredini R, Tagliafico E, Zini R, Jones A, Zoi K, Reiter A, Duncombe A, Pietra D, Rumi E, Cervantes F, Barosi G, Cazzola M, Cross NC, Tefferi A. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861–1869. doi: 10.1038/leu.2013.119. - DOI - PubMed
-
- Palandri F, Palumbo GA, Iurlo A, Polverelli N, Benevolo G, Breccia M, Abruzzese E, Tiribelli M, Bonifacio M, Tieghi A, Isidori A, Martino B, Sgherza N, D’Adda M, Bergamaschi M, Crugnola M, Cavazzini F, Bosi C, Binotto G, Auteri G, Latagliata R, Ibatici A, Scaffidi L, Penna D, Cattaneo D, Soci F, Trawinska M, Russo D, Cuneo A, Semenzato G, Di Raimondo F, Aversa F, Lemoli RM, Heidel F, Reggiani MLB, Bartoletti D, Cavo M, Catani L, Vianelli N. Differences in presenting features, outcome and prognostic models in patients with primary myelofibrosis and post-polycythemia vera and/or post-essential thrombocythemia myelofibrosis treated with ruxolitinib. New perspective of the MYSEC-PM in a large multicenter study. Semin Hematol. 2018;55(4):248–255. doi: 10.1053/j.seminhematol.2018.05.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

