Helicobacter pylori CagA oncoprotein interacts with SHIP2 to increase its delivery into gastric epithelial cells
- PMID: 32198795
- PMCID: PMC7226221
- DOI: 10.1111/cas.14391
Helicobacter pylori CagA oncoprotein interacts with SHIP2 to increase its delivery into gastric epithelial cells
Abstract
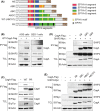
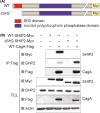
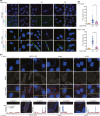
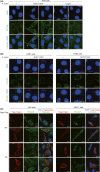
Chronic infection with Helicobacter pylori cagA-positive strains is causally associated with the development of gastric diseases, most notably gastric cancer. The cagA-encoded CagA protein, which is injected into gastric epithelial cells by bacterial type IV secretion, undergoes tyrosine phosphorylation at the Glu-Pro-Ile-Tyr-Ala (EPIYA) segments (EPIYA-A, EPIYA-B, EPIYA-C, and EPIYA-D), which are present in various numbers and combinations in its C-terminal polymorphic region, thereby enabling CagA to promiscuously interact with SH2 domain-containing host cell proteins, including the prooncogenic SH2 domain-containing protein tyrosine phosphatase 2 (SHP2). Perturbation of host protein functions by aberrant complex formation with CagA has been considered to contribute to the development of gastric cancer. Here we show that SHIP2, an SH2 domain-containing phosphatidylinositol 5'-phosphatase, is a hitherto undiscovered CagA-binding host protein. Similar to SHP2, SHIP2 binds to the Western CagA-specific EPIYA-C segment or East Asian CagA-specific EPIYA-D segment through the SH2 domain in a tyrosine phosphorylation-dependent manner. In contrast to the case of SHP2, however, SHIP2 binds more strongly to EPIYA-C than to EPIYA-D. Interaction with CagA tethers SHIP2 to the plasma membrane, where it mediates production of phosphatidylinositol 3,4-diphosphate [PI(3,4)P2 ]. The CagA-SHIP2 interaction also potentiates the morphogenetic activity of CagA, which is caused by CagA-deregulated SHP2. This study indicates that initially delivered CagA interacts with SHIP2 and thereby strengthens H. pylori-host cell attachment by altering membrane phosphatidylinositol compositions, which potentiates subsequent delivery of CagA that binds to and thereby deregulates the prooncogenic phosphatase SHP2.
Keywords: Helicobacter pylori; CagA; PI(3,4)P2; SHIP2; gastric cancer.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Blaser MJ, Perez‐Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111‐2115. - PubMed
-
- Higashi H, Tsutsumi R, Muto S, et al. SHP‐2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683‐686. - PubMed
-
- Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688‐694. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

