Modeling Favipiravir Antiviral Efficacy Against Emerging Viruses: From Animal Studies to Clinical Trials
- PMID: 32198838
- PMCID: PMC7239338
- DOI: 10.1002/psp4.12510
Modeling Favipiravir Antiviral Efficacy Against Emerging Viruses: From Animal Studies to Clinical Trials
Abstract
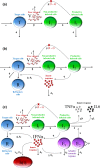
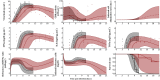
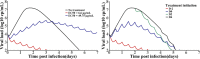
In 2014, our research network was involved in the evaluation of favipiravir, an anti-influenza polymerase inhibitor, against Ebola virus. In this review, we discuss how mathematical modeling was used, first to propose a relevant dosing regimen in humans, and then to optimize its antiviral efficacy in a nonhuman primate (NHP) model. The data collected in NHPs were finally used to develop a model of Ebola pathogenesis integrating the interactions among the virus, the innate and adaptive immune response, and the action of favipiravir. We conclude the review of this work by discussing how these results are of relevance for future human studies in the context of Ebola virus, but also for other emerging viral diseases for which no therapeutics are available.
© 2020 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
As Editor‐in‐Chief for
Figures




References
-
- Marston, H.D. , Folkers, G.K. , Morens, D.M. & Fauci, A.S. Emerging viral diseases: confronting threats with new technologies. Sci. Transl. Med. 6, 253ps10–253ps10 (2014). - PubMed
-
- Warren, T. et al Efficacy of galidesivir against Ebola virus disease in rhesus monkeys. Open Forum Infect. Dis. 4, S302 (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

