Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer's disease: Evidence of ferroptosis
- PMID: 32199332
- PMCID: PMC7083890
- DOI: 10.1016/j.redox.2020.101494
Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer's disease: Evidence of ferroptosis
Abstract
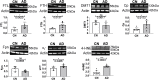
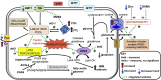
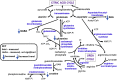
Iron dyshomeostasis is implicated in Alzheimer's disease (AD) alongside β-amyloid and tau pathologies. Despite the recent discovery of ferroptosis, an iron-dependent form cell death, hitherto, in vivo evidence of ferroptosis in AD is lacking. The present study uniquely adopts an integrated multi-disciplinary approach, combining protein (Western blot) and elemental analysis (total reflection X-ray fluorescence) with metabolomics (1H nuclear magnetic resonance spectroscopy) to identify iron dyshomeostasis and ferroptosis, and possible novel interactions with metabolic dysfunction in age-matched male cognitively normal (CN) and AD post-mortem brain tissue (n = 7/group). Statistical analysis was used to compute differences between CN and AD, and to examine associations between proteins, elements and/or metabolites. Iron dyshomeostasis with elevated levels of ferritin, in the absence of increased elemental iron, was observed in AD. Moreover, AD was characterised by enhanced expression of the light-chain subunit of the cystine/glutamate transporter (xCT) and lipid peroxidation, reminiscent of ferroptosis, alongside an augmented excitatory glutamate to inhibitory GABA ratio. Protein, element and metabolite associations also greatly differed between CN and AD suggesting widespread metabolic dysregulation in AD. We demonstrate iron dyshomeostasis, upregulated xCT (impaired glutathione metabolism) and lipid peroxidation in AD, suggesting anti-ferroptotic therapies may be efficacious in AD.
Keywords: Alzheimer’s disease; Excitotoxicity; Ferroptosis; Glutamate/cystine antiporter; Iron dyshomeostasis; Lipid peroxidation.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

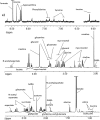
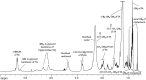

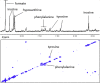


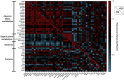
 C) refers to quantification of –CH2-CH
C) refers to quantification of –CH2-CH CH-CH2- of fatty acid chains.
CH-CH2- of fatty acid chains.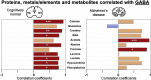

References
-
- Benarroch E.E. Brain iron homeostasis and neurodegenerative disease. Neurology. 2009;72(16):1436–1440. - PubMed
-
- Bartzokis G., Sultzer D., Mintz J., et al. In vivo evaluation of brain iron in Alzheimer's disease and normal subjects using MRI. Biol. Psychiatr. 1994;35(7):480–487. - PubMed
-
- Selkoe D.J. Alzheimer disease and aducanumab: adjusting our approach. Nat. Rev. Neurol. 2019;15(7):365–366. - PubMed
-
- Mintun M.A., Larossa G.N., Sheline Y.I., et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

