Carcinogenesis of Pancreatic Ductal Adenocarcinoma
- PMID: 32199881
- PMCID: PMC7282937
- DOI: 10.1053/j.gastro.2020.02.059
Carcinogenesis of Pancreatic Ductal Adenocarcinoma
Abstract
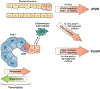
Although the estimated time for development of pancreatic ductal adenocarcinoma (PDA) is more than 20 years, PDAs are usually detected at late, metastatic stages. PDAs develop from duct-like cells through a multistep carcinogenesis process, from low-grade dysplastic lesions to carcinoma in situ and eventually to metastatic disease. This process involves gradual acquisition of mutations in oncogenes and tumor suppressor genes, as well as changes in the pancreatic environment from a pro-inflammatory microenvironment that favors the development of early lesions, to a desmoplastic tumor microenvironment that is highly fibrotic and immune suppressive. This review discusses our current understanding of how PDA originates.
Keywords: Carcinogenesis; Desmoplastic Reaction; Microenvironment; Pancreatic Ductal Adenocarcinoma; Pancreatic Intraepithelial Neoplasia.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest:
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter discussed in this manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

