IL-5Rα marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease
- PMID: 32199912
- PMCID: PMC7282948
- DOI: 10.1016/j.jaci.2020.02.035
IL-5Rα marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease
Abstract
Background: The cause of severe nasal polyposis in aspirin-exacerbated respiratory disease (AERD) is unknown. Elevated antibody levels have been associated with disease severity in nasal polyps, but upstream drivers of local antibody production in nasal polyps are undetermined.
Objective: We sought to identify upstream drivers and phenotypic properties of local antibody-expressing cells in nasal polyps from subjects with AERD.
Methods: Sinus tissue was obtained from subjects with AERD, chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP), CRS without nasal polyps, and controls without CRS. Tissue antibody levels were quantified via ELISA and immunohistochemistry and were correlated with disease severity. Antibody-expressing cells were profiled with single-cell RNA sequencing, flow cytometry, and immunofluorescence, with IL-5Rα function determined through IL-5 stimulation and subsequent RNA sequencing and quantitative PCR.
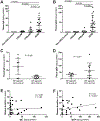
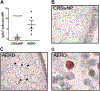
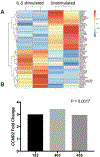
Results: Tissue IgE and IgG4 levels were elevated in AERD compared with in controls (P < .01 for IgE and P < .001 for IgG4 vs CRSwNP). Subjects with AERD whose nasal polyps recurred rapidly had higher IgE levels than did subjects with AERD, with slower regrowth (P = .005). Single-cell RNA sequencing revealed increased IL5RA, IGHG4, and IGHE in antibody-expressing cells from patients with AERD compared with antibody-expressing cells from patients with CRSwNP. There were more IL-5Rα+ plasma cells in the polyp tissue from those with AERD than in polyp tissue from those with CRSwNP (P = .026). IL-5 stimulation of plasma cells in vitro induced changes in a distinct set of transcripts.
Conclusions: Our study identifies an increase in antibody-expressing cells in AERD defined by transcript enrichment of IL5RA and IGHG4 or IGHE, with confirmed surface expression of IL-5Rα and functional IL-5 signaling. Tissue IgE and IgG4 levels are elevated in AERD, and higher IgE levels are associated with faster nasal polyp regrowth. Our findings suggest a role for IL-5Rα+ antibody-expressing cells in facilitating local antibody production and severe nasal polyps in AERD.
Keywords: Aspirin-exacerbated respiratory disease; IL-5Rα; IgE; IgG4; chronic rhinosinusitis; interleukin-5; nasal polyposis; plasma cell.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: K.M. Buchheit has received compensation for scientific advisory boards from Regeneron, Genentech, and AstraZeneca. A.K. Shalek has received compensation for consulting fees and scientific advisory board membership from Honeycomb Biotechnologies, Dot Bio, Cellarity, Cogen Therapeutics, and Dahlia Biosciences. N.A. Barrett has received compensation for consulting fees from Regeneron. T.M. Laidlaw has received compensation for consulting fees and scientific advisory board membership from Novartis, Regeneron, and Sanofi-Genzyme. The rest of the authors declare no conflicts of interest.
Figures


Similar articles
-
Nasal polyp antibody-secreting cells display proliferation signature in aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2024 Feb;153(2):527-532. doi: 10.1016/j.jaci.2023.10.011. Epub 2023 Oct 28. J Allergy Clin Immunol. 2024. PMID: 37898408 Free PMC article.
-
Transcriptome Analysis Identifies Doublesex and Mab-3 Related Transcription Factor (DMRT3) in Nasal Polyp Epithelial Cells of Patients Suffering from Non-Steroidal Anti-Inflammatory Drug-Exacerbated Respiratory Disease (AERD).Biomolecules. 2021 Jul 23;11(8):1092. doi: 10.3390/biom11081092. Biomolecules. 2021. PMID: 34439758 Free PMC article.
-
Mepolizumab targets multiple immune cells in aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2021 Aug;148(2):574-584. doi: 10.1016/j.jaci.2021.05.043. Epub 2021 Jun 16. J Allergy Clin Immunol. 2021. PMID: 34144111 Free PMC article. Clinical Trial.
-
New concepts for the pathogenesis and management of aspirin-exacerbated respiratory disease.Curr Opin Allergy Clin Immunol. 2022 Feb 1;22(1):42-48. doi: 10.1097/ACI.0000000000000795. Curr Opin Allergy Clin Immunol. 2022. PMID: 34739410 Free PMC article. Review.
-
Aspirin-exacerbated respiratory disease and current treatment modalities.Eur Arch Otorhinolaryngol. 2017 Mar;274(3):1291-1300. doi: 10.1007/s00405-016-4273-1. Epub 2016 Aug 18. Eur Arch Otorhinolaryngol. 2017. PMID: 27538737 Review.
Cited by
-
Local and Systemic Production of Pro-Inflammatory Eicosanoids Is Inversely Related to Sensitization to Aeroallergens in Patients with Aspirin-Exacerbated Respiratory Disease.J Pers Med. 2022 Mar 11;12(3):447. doi: 10.3390/jpm12030447. J Pers Med. 2022. PMID: 35330446 Free PMC article.
-
Use of nCounter mRNA profiling to identify at-arrival gene expression patterns for predicting bovine respiratory disease in beef cattle.BMC Vet Res. 2022 Feb 23;18(1):77. doi: 10.1186/s12917-022-03178-8. BMC Vet Res. 2022. PMID: 35197051 Free PMC article.
-
Clinical efficacy and mechanisms of biologics for chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2025 May;155(5):1401-1410. doi: 10.1016/j.jaci.2025.03.011. Epub 2025 Mar 23. J Allergy Clin Immunol. 2025. PMID: 40132672 Review.
-
Characteristics of mucin hypersecretion in different inflammatory patterns based on endotypes of chronic rhinosinusitis.Clin Transl Allergy. 2024 Jan;14(1):e12334. doi: 10.1002/clt2.12334. Clin Transl Allergy. 2024. PMID: 38282195 Free PMC article. Review.
-
B Lineage Cells and IgE in Allergic Rhinitis and CRSwNP and the Role of Omalizumab Treatment.Am J Rhinol Allergy. 2023 Mar;37(2):182-192. doi: 10.1177/19458924221147770. Am J Rhinol Allergy. 2023. PMID: 36848269 Free PMC article. Review.
References
-
- Bhattacharyya N Influence of polyps on outcomes after endoscopic sinus surgery. The Laryngoscope. 2007;117(10):1834–8. - PubMed
-
- Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: A systematic review. The Laryngoscope. 2015;125(7):1547–56. - PubMed
-
- Bhattacharyya N Assessing the additional disease burden of polyps in chronic rhinosinusitis. The Annals of otology, rhinology, and laryngology. 2009;118(3):185–9. - PubMed
-
- Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. The Journal of allergy and clinical immunology. 2003;111(4):743–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL128241/HL/NHLBI NIH HHS/United States
- R01 HL095791/HL/NHLBI NIH HHS/United States
- R01 AI134989/AI/NIAID NIH HHS/United States
- K08 AI132723/AI/NIAID NIH HHS/United States
- R01 HL117945/HL/NHLBI NIH HHS/United States
- U19 AI095219/AI/NIAID NIH HHS/United States
- R01 AI078908/AI/NIAID NIH HHS/United States
- K23 HL111113/HL/NHLBI NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R37 AI052353/AI/NIAID NIH HHS/United States
- RM1 HG006193/HG/NHGRI NIH HHS/United States
- K23 AI139352/AI/NIAID NIH HHS/United States
- R01 HL134539/HL/NHLBI NIH HHS/United States
- R01 AI138546/AI/NIAID NIH HHS/United States
- T32 AI007306/AI/NIAID NIH HHS/United States
- R01 HL136209/HL/NHLBI NIH HHS/United States
- U19 AI089992/AI/NIAID NIH HHS/United States
- U24 AI118672/AI/NIAID NIH HHS/United States
- L30 AI126566/AI/NIAID NIH HHS/United States
- R01 AI136041/AI/NIAID NIH HHS/United States
- DRG-2274-16/HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

