RGS4 promotes allergen- and aspirin-associated airway hyperresponsiveness by inhibiting PGE2 biosynthesis
- PMID: 32199913
- PMCID: PMC7501178
- DOI: 10.1016/j.jaci.2020.03.004
RGS4 promotes allergen- and aspirin-associated airway hyperresponsiveness by inhibiting PGE2 biosynthesis
Abstract
Background: Allergens elicit host production of mediators acting on G-protein-coupled receptors to regulate airway tone. Among these is prostaglandin E2 (PGE2), which, in addition to its role as a bronchodilator, has anti-inflammatory actions. Some patients with asthma develop bronchospasm after the ingestion of aspirin and other nonsteroidal anti-inflammatory drugs, a disorder termed aspirin-exacerbated respiratory disease. This condition may result in part from abnormal dependence on the bronchoprotective actions of PGE2.
Objective: We sought to understand the functions of regulator of G protein signaling 4 (RGS4), a cytoplasmic protein expressed in airway smooth muscle and bronchial epithelium that regulates the activity of G-protein-coupled receptors, in asthma.
Methods: We examined RGS4 expression in human lung biopsies by immunohistochemistry. We assessed airways hyperresponsiveness (AHR) and lung inflammation in germline and airway smooth muscle-specific Rgs4-/- mice and in mice treated with an RGS4 antagonist after challenge with Aspergillus fumigatus. We examined the role of RGS4 in nonsteroidal anti-inflammatory drug-associated bronchoconstriction by challenging aspirin-exacerbated respiratory disease-like (ptges1-/-) mice with aspirin.
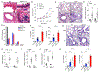
Results: RGS4 expression in respiratory epithelium is increased in subjects with severe asthma. Allergen-induced AHR was unexpectedly diminished in Rgs4-/- mice, a finding associated with increased airway PGE2 levels. RGS4 modulated allergen-induced PGE2 secretion in human bronchial epithelial cells and prostanoid-dependent bronchodilation. The RGS4 antagonist CCG203769 attenuated AHR induced by allergen or aspirin challenge of wild-type or ptges1-/- mice, respectively, in association with increased airway PGE2 levels.
Conclusions: RGS4 may contribute to the development of AHR by reducing airway PGE2 biosynthesis in allergen- and aspirin-induced asthma.
Keywords: Asthma; G proteins; PGE2; aspirin sensitivity; aspirin-exacerbated respiratory disease; regulators of G protein signaling protein.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



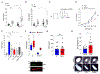



References
-
- Lambrecht BN, Hammad H, and Fahy JV. The Cytokines of Asthma. Immunity. 2019;50(4):975–91. - PubMed
-
- Boonpiyathad T, Sozener ZC, Satitsuksanoa P, and Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. 2019;46:101333. - PubMed
-
- Gon Y, and Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int. 2018;67(1):12–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

