Dynamic treatment regimens in small n, sequential, multiple assignment, randomized trials: An application in focal segmental glomerulosclerosis
- PMID: 32200006
- PMCID: PMC8173713
- DOI: 10.1016/j.cct.2020.105989
Dynamic treatment regimens in small n, sequential, multiple assignment, randomized trials: An application in focal segmental glomerulosclerosis
Abstract
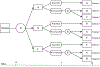
Focal segmental glomerulosclerosis (FSGS) is a rare kidney disease with an annual incidence of 0.2-1.8 cases per 100,000 individuals. Most rare diseases like FSGS lack effective treatments, and it is difficult to implement clinical trials to study rare diseases because of the small sample sizes and difficulty in recruitment. A novel clinical trial design, a small sample, sequential, multiple assignment, randomized trial (snSMART) has been proposed to efficiently identify effective treatments for rare diseases. In this work, we review and expand the snSMART design applied to studying treatments for FSGS. The snSMART is a multistage trial that randomizes participants to one of three active treatments in the first stage and then re-randomizes those who do not respond to the initial treatment to one of the other two treatments in the second stage. A Bayesian joint stage model efficiently shares information across the stages to find the best first stage treatment. In this setting, we modify the previously presented design and methods (Wei et al. 2018) such that the proposed design includes a standard of care as opposed to three active treatments. We present Bayesian and frequentist models to compare the two novel therapies to the standard of care. Additionally, we show for the first time how we should estimate and compare tailored sequences of treatments or dynamic treatment regimens (DTRs) and contrast the results from our methods to existing methods for analyzing DTRs from a SMART. We also propose a sample size calculation method for our snSMART design when implementing the frequentist model with Dunnett's correction.
Keywords: Clinical trial; Effect estimation; Small sample size.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

References
-
- D’Agati VD, Kaskel FJ, Falk RJ, Focal segmental glomerulosclerosis N. Engl. J. Med 365 (25) (2011) 2398–2411. - PubMed
-
- Hsu JC, The factor analytic approach to simultaneous inference in the general linear model, J. Comput. Graph. Stat 1 (2) (1992) 151–168.
-
- Mancl LA, DeRouen TA, A covariance estimator for GEE with improved small-sample properties, Biometrics 57 (2001) 126–134. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

