Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials
- PMID: 32200512
- PMCID: PMC7211779
- DOI: 10.1007/s13555-020-00367-x
Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials
Abstract
Introduction: Ixekizumab, a high-affinity monoclonal antibody that selectively targets interleukin-17A, is approved for treatment of moderate-to-severe plaque psoriasis. Our objective was to evaluate the long-term efficacy and safety of ixekizumab in moderate-to-severe plaque psoriasis through 5 years.
Methods: Data were integrated from the UNCOVER-1 and UNCOVER-2, randomized, double-blinded, phase-3 trials. Patients who continuously received the labeled ixekizumab dose, were static Physician's Global Assessment (sPGA) (0,1) responders at Week 12 and completed 60 weeks of treatment could enter the long-term extension (LTE) period. Patients could escalate to every-2-week dosing per investigator opinion. Efficacy and health outcomes included proportion of patients achieving Psoriasis Area and Severity Index (PASI) 75/90/100, sPGA (0,1) and (0), absolute PASI ≤ 5/ ≤ 3/ ≤ 2/ ≤ 1 and Dermatology Life Quality Index (DLQI) (0,1). Results exclude patients who escalated to every-2-week dosing. A modified non-responder imputation method was used to account for missing data. Supplemental analyses include patients who escalated to every-2-week dosing and observed and multiple imputation results. Exposure-adjusted safety outcomes are also reported.
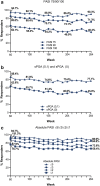
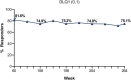
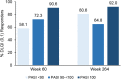
Results: Of 206 patients who entered the LTE periods, 172 completed treatment. At Week 60, PASI 75/90/100 responses were 94.7%, 85.0% and 62.1%, respectively, and at year 5 were 90.3%, 71.3% and 46.3%, respectively. Similarly, meaningful responses were achieved for the other efficacy and health measures. Among patients with PASI 100 through 5 years, 92% achieved DLQI (0,1), indicating no impact of skin disease on quality of life. During the LTE period, exposure-adjusted incidence rates were 31.4 per 100 patient-years for treatment-emergent adverse events and 6.8 per 100 patient-years for serious adverse events. No deaths were reported. No new or unexpected safety findings were noted.
Conclusions: The results demonstrate 80 mg ixekizumab maintains long-term efficacy and a safety profile consistent with previous data in patients with moderate-to-severe plaque psoriasis through 5 years of treatment.
Trial registration: ClinicalTrials.gov identifier, UNCOVER-1: NCT01474512, UNCOVER-2: NCT01597245.
Keywords: 5 years; Ixekizumab; Long-term efficacy; Long-term safety; Maintain; Psoriasis; Quality of life.
Figures

References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. - PubMed
-
- Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332:581–588. - PubMed
-
- World Health Organization . WHO global report on psoriasis. Switzerland: WHO Press; 2016.
-
- Lewis-Beck C, Abouzaid S, Xie L, Baser O, Kim E. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer Adherence. 2013;7:199–205. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

