Glucose Uptake and Insulin Response in Tissue-engineered Human Skeletal Muscle
- PMID: 32200516
- PMCID: PMC7710786
- DOI: 10.1007/s13770-020-00242-y
Glucose Uptake and Insulin Response in Tissue-engineered Human Skeletal Muscle
Abstract
Background: Tissue-engineered muscles ("myobundles") offer a promising platform for developing a human in vitro model of healthy and diseased muscle for drug development and testing. Compared to traditional monolayer cultures, myobundles better model the three-dimensional structure of native skeletal muscle and are amenable to diverse functional measures to monitor the muscle health and drug response. Characterizing the metabolic function of human myobundles is of particular interest to enable their utilization in mechanistic studies of human metabolic diseases, identification of related drug targets, and systematic studies of drug safety and efficacy.
Methods: To this end, we studied glucose uptake and insulin responsiveness in human tissue-engineered skeletal muscle myobundles in the basal state and in response to drug treatments.
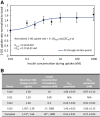
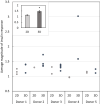
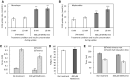
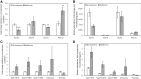
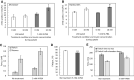
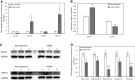
Results: In the human skeletal muscle myobundle system, insulin stimulates a 50% increase in 2-deoxyglucose (2-DG) uptake with a compiled EC50 of 0.27 ± 0.03 nM. Treatment of myobundles with 400 µM metformin increased basal 2-DG uptake 1.7-fold and caused a significant drop in twitch and tetanus contractile force along with decreased fatigue resistance. Treatment with the histone deacetylase inhibitor 4-phenylbutyrate (4-PBA) increased the magnitude of insulin response from a 1.2-fold increase in glucose uptake in the untreated state to a 1.4-fold increase after 4-PBA treatment. 4-PBA treated myobundles also exhibited increased fatigue resistance and increased twitch half-relaxation time.
Conclusion: Although tissue-engineered human myobundles exhibit a modest increase in glucose uptake in response to insulin, they recapitulate key features of in vivo insulin sensitivity and exhibit relevant drug-mediated perturbations in contractile function and glucose metabolism.
Keywords: Insulin; Muscle; Myobundles; Skeletal; Tissue engineering.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
Similar articles
-
Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle.Biomaterials. 2019 Apr;198:259-269. doi: 10.1016/j.biomaterials.2018.08.058. Epub 2018 Aug 31. Biomaterials. 2019. PMID: 30180985 Free PMC article.
-
Microphysiological system for studying contractile differences in young, active, and old, sedentary adult derived skeletal muscle cells.Aging Cell. 2022 Jul;21(7):e13650. doi: 10.1111/acel.13650. Epub 2022 Jun 2. Aging Cell. 2022. PMID: 35653714 Free PMC article.
-
Human, Tissue-Engineered, Skeletal Muscle Myobundles to Measure Oxygen Uptake and Assess Mitochondrial Toxicity.Tissue Eng Part C Methods. 2017 Apr;23(4):189-199. doi: 10.1089/ten.tec.2016.0264. Epub 2017 Mar 24. Tissue Eng Part C Methods. 2017. PMID: 28338413 Free PMC article.
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
Growth Factors for Skeletal Muscle Tissue Engineering.Cells Tissues Organs. 2016;202(3-4):169-179. doi: 10.1159/000444671. Epub 2016 Nov 9. Cells Tissues Organs. 2016. PMID: 27825154 Free PMC article. Review.
Cited by
-
Modeling statin myopathy in a human skeletal muscle microphysiological system.PLoS One. 2020 Nov 25;15(11):e0242422. doi: 10.1371/journal.pone.0242422. eCollection 2020. PLoS One. 2020. PMID: 33237943 Free PMC article.
-
Human Myobundle Platform for Studying the Role of Notch Signaling in Satellite Cell Phenotype and Function.Adv Healthc Mater. 2025 May;14(12):e2404695. doi: 10.1002/adhm.202404695. Epub 2025 Mar 24. Adv Healthc Mater. 2025. PMID: 40123310
-
Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Glucose Intolerance.Curr Issues Mol Biol. 2024 Jan 24;46(2):1078-1090. doi: 10.3390/cimb46020068. Curr Issues Mol Biol. 2024. PMID: 38392186 Free PMC article.
-
Adipogenic Differentiation Alters Properties of Vascularized Tissue-Engineered Skeletal Muscle.Tissue Eng Part A. 2022 Jan;28(1-2):54-68. doi: 10.1089/ten.TEA.2021.0064. Epub 2021 Aug 25. Tissue Eng Part A. 2022. PMID: 34102861 Free PMC article.
-
Myoblast deactivation within engineered human skeletal muscle creates a transcriptionally heterogeneous population of quiescent satellite-like cells.Biomaterials. 2022 May;284:121508. doi: 10.1016/j.biomaterials.2022.121508. Epub 2022 Apr 7. Biomaterials. 2022. PMID: 35421801 Free PMC article.
References
-
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985). 2000;89:81–88. - PubMed
-
- Latroche C, Gitiaux C, Chrétien F, Desguerre I, Mounier R, Chazaud B. Skeletal muscle microvasculature: a highly dynamic lifeline. Physiology (Bethesda). 2015;30:417–427. - PubMed
-
- Collinsworth AM, Zhang S, Kraus WE, Truskey GA. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol. 2002;283:C1219–C1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
