Expanded Access as a source of real-world data: An overview of FDA and EMA approvals
- PMID: 32200551
- PMCID: PMC7444779
- DOI: 10.1111/bcp.14284
Expanded Access as a source of real-world data: An overview of FDA and EMA approvals
Abstract
Aims: To identify, characterize and compare all Food and Drug Administration (FDA) and European Medicines Agency (EMA) approvals that included real-world data on efficacy from expanded access (EA) programmes.
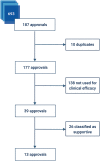
Methods: Cross-sectional study of FDA (1955-2018) and EMA (1995-2018) regulatory approval documentation. We automated searching for terms related to EA in 22,506 documents using machine learning techniques. We included all approvals where EA terms appeared in the regulatory documentation. Our main outcome was the inclusion of EA data as evidence of clinical efficacy. Characterization was based on approval date, disease area, orphan designation and whether the evidence was supportive or pivotal.
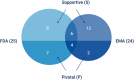
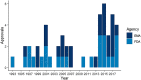
Results: EA terms appeared in 693 out of 22,506 (3.1%) documents, which referenced 187 approvals. For 39 approvals, data from EA programmes were used to inform on clinical efficacy. The yearly number of approvals with EA data increased from 1.25 for 1993-2013 to 4.6 from 2014-2018. In 13 cases, these programmes formed the main evidence for approval. Of these, patients in EA programmes formed over half (median 71%, interquartile range: 34-100) of the total patient population available for efficacy evaluation. Almost all (12/13) approvals were granted orphan designation. In 8/13, there were differences between regulators in approval status and valuation of evidence. Strikingly, 4 treatments were granted approval based solely on efficacy from EA.
Conclusion: Sponsors and regulators increasingly include real-world data from EA programmes in the efficacy profile of a treatment. The indications of the approved treatments are characterized by orphan designation and high unmet medical need.
Keywords: drug regulation; effectiveness; evidence-based medicine; health policy.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Tobias Polak received an unrestricted grant from myTomorrows for a part‐time doctorate trajectory. Tobias Polak works part‐time as an RWD lead for myTomorrows. Joost van Rosmalen declares no conflict of interest. Carin Uyl‐de Groot has received unrestricted grants from Boehringer Ingelheim, Astellas, Celgene, Sanofi, Janssen‐Cilag, Bayer, Amgen, Genzyme, Merck, Glycostem Therapeutics, Astra Zeneca, Roche and Merck.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

