Neuroinflammation Mediated by Glia Maturation Factor Exacerbates Neuronal Injury in an in vitro Model of Traumatic Brain Injury
- PMID: 32200671
- PMCID: PMC7336883
- DOI: 10.1089/neu.2019.6932
Neuroinflammation Mediated by Glia Maturation Factor Exacerbates Neuronal Injury in an in vitro Model of Traumatic Brain Injury
Abstract
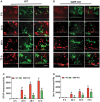
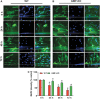
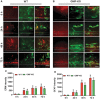
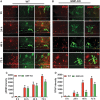
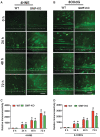
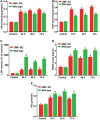
Traumatic brain injury (TBI) is the primary cause of death and disability affecting over 10 million people in the industrialized world. TBI causes a wide spectrum of secondary molecular and cellular complications in the brain. However, the pathological events are still not yet fully understood. Previously, we have shown that the glia maturation factor (GMF) is a mediator of neuroinflammation in neurodegenerative diseases. To identify the potential molecular pathways accompanying TBI, we used an in vitro cell culture model of TBI. A standardized injury was induced by scalpel cut through a mixed primary cell culture of astrocytes, microglia and neurons obtained from both wild type (WT) and GMF-deficient (GMF-KO) mice. Cell culture medium and whole-cell lysates were collected at 24, 48, and 72 h after the scalpel cuts injury and probed for oxidative stress using immunofluorescence analysis. Results showed that oxidative stress markers such as glutathione and glutathione peroxidase were significantly reduced, while release of cytosolic enzyme lactate dehydrogenase along with nitric oxide and prostaglandin E2 were significantly increased in injured WT cells compared with injured GMF-KO cells. In addition, injured WT cells showed increased levels of oxidation product 4-hydroxynonenal and 8-oxo-2'-deoxyguanosine compared with injured GMF-KO cells. Further, we found that injured WT cells showed a significantly increased expression of glial fibrillary acidic protein, ionized calcium binding adaptor molecule 1, and phosphorylated ezrin/radixin/moesin proteins, and reduced microtubule associated protein expression compared with injured GMF-KO cells after injury. Collectively, our results demonstrate that GMF exacerbates the oxidative stress-mediated neuroinflammation that could be brought about by TBI-induced astroglial activation.
Keywords: ezrin/radixin/moesin proteins; glia maturation factor; neurodegeneration; neuroinflammation; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Figures
References
-
- Stahel P.F., Shohami E., Younis F.M., Kariya K., Otto V.I., Lenzlinger P.M., Grosjean M.B., Eugster H.P., Trentz O., Kossmann T., and Morganti-Kossmann M.C. (2000). Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb Blood Flow Metab. 20, 369–380 - PubMed
-
- Hang C.H., Shi J.X., Li J.S., Wu W., and Yin H.X. (2005). Concomitant upregulation of nuclear factor-kB activity, proinflammatory cytokines and ICAM-1 in the injured brain after cortical contusion trauma in a rat model. Neurol. India 53, 312–317 - PubMed
-
- Jin W., Zhu L., Guan Q., Chen G., Wang Q.F., Yin H.X., Hang C.H., Shi J.X., and Wang H.D. (2008). Influence of Nrf2 genotype on pulmonary NF-kappaB activity and inflammatory response after traumatic brain injury. Ann. Clin. Lab. Sci. 38, 221–227 - PubMed
-
- Morrison B., 3rd, Elkin B.S., Dolle J.P., and Yarmush M.L. (2011). In vitro models of traumatic brain injury. Annu. Rev. Biomed. Eng. 13, 91–126 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

