Endothelin-1 Mediates the Systemic and Renal Hemodynamic Effects of GPR81 Activation
- PMID: 32200679
- PMCID: PMC7176350
- DOI: 10.1161/HYPERTENSIONAHA.119.14308
Endothelin-1 Mediates the Systemic and Renal Hemodynamic Effects of GPR81 Activation
Abstract
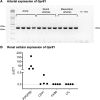
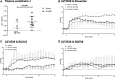
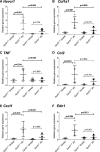
GPR81 (G-protein-coupled receptor 81) is highly expressed in adipocytes, and activation by the endogenous ligand lactate inhibits lipolysis. GPR81 is also expressed in the heart, liver, and kidney, but roles in nonadipose tissues are poorly defined. GPR81 agonists, developed to improve blood lipid profile, might also provide insights into GPR81 physiology. Here, we assessed the blood pressure and renal hemodynamic responses to the GPR81 agonist, AZ'5538. In male wild-type mice, intravenous AZ'5538 infusion caused a rapid and sustained increase in systolic and diastolic blood pressure. Renal artery blood flow, intrarenal tissue perfusion, and glomerular filtration rate were all significantly reduced. AZ'5538 had no effect on blood pressure or renal hemodynamics in Gpr81-/- mice. Gpr81 mRNA was expressed in renal artery vascular smooth muscle, in the afferent arteriole, in glomerular and medullary perivascular cells, and in pericyte-like cells isolated from kidney. Intravenous AZ'5538 increased plasma ET-1 (endothelin 1), and pretreatment with BQ123 (endothelin-A receptor antagonist) prevented the pressor effects of GPR81 activation, whereas BQ788 (endothelin-B receptor antagonist) did not. Renal ischemia-reperfusion injury, which increases renal extracellular lactate, increased the renal expression of genes encoding ET-1, KIM-1 (Kidney Injury Molecule 1), collagen type 1-α1, TNF-α (tumor necrosis factor-α), and F4/80 in wild-type mice but not in Gpr81-/- mice. In summary, activation of GPR81 in vascular smooth muscle and perivascular cells regulates renal hemodynamics, mediated by release of the potent vasoconstrictor ET-1. This suggests that lactate may be a paracrine regulator of renal blood flow, particularly relevant when extracellular lactate is high as occurs during ischemic renal disease.
Keywords: blood pressure; hypoxia; ischemia-reperfusion-injury; pericytes; renal blood flow.
Figures



References
-
- Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009;30:557–562. doi: 10.1016/j.tips.2009.09.001. - PubMed
-
- Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex. 2014;24:2784–2795. doi: 10.1093/cercor/bht136. - PubMed
-
- Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, Wright SD, Taggart AK, Waters MG. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun. 2008;377:987–991. doi: 10.1016/j.bbrc.2008.10.088. - PubMed
-
- Chudalla R, Baerwalde S, Schneider G, Maassen N. Local and systemic effects on blood lactate concentration during exercise with small and large muscle groups. Pflugers Arch. 2006;452:690–697. doi: 10.1007/s00424-006-0082-5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

