Mitochondrial Functions in Infection and Immunity
- PMID: 32200805
- PMCID: PMC7126537
- DOI: 10.1016/j.tcb.2020.01.006
Mitochondrial Functions in Infection and Immunity
Erratum in
-
Mitochondrial Functions in Infection and Immunity: (Trends in Cell Biology 30, 263-275, 2020).Trends Cell Biol. 2020 Sep;30(9):748. doi: 10.1016/j.tcb.2020.07.001. Epub 2020 Jul 20. Trends Cell Biol. 2020. PMID: 32703641 Free PMC article. No abstract available.
Abstract
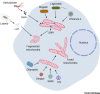
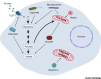
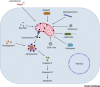
Mitochondria have a central role in regulating a range of cellular activities and host responses upon bacterial infection. Multiple pathogens affect mitochondria dynamics and functions to influence their intracellular survival or evade host immunity. On the other side, major host responses elicited against infections are directly dependent on mitochondrial functions, thus placing mitochondria centrally in maintaining homeostasis upon infection. In this review, we summarize how different bacteria and viruses impact morphological and functional changes in host mitochondria and how this manipulation can influence microbial pathogenesis as well as the host cell metabolism and immune responses.
Keywords: bacteria; cell death; innate immunity; mitochondrial metabolism; mitochondrial morphology; viruses.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Roger A.J. The origin and diversification of mitochondria. Curr. Biol. 2017;27:R1177–R1192. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

