Genome-wide Association Analysis of Parkinson's Disease and Schizophrenia Reveals Shared Genetic Architecture and Identifies Novel Risk Loci
- PMID: 32201043
- PMCID: PMC7416467
- DOI: 10.1016/j.biopsych.2020.01.026
Genome-wide Association Analysis of Parkinson's Disease and Schizophrenia Reveals Shared Genetic Architecture and Identifies Novel Risk Loci
Abstract
Background: Parkinson's disease (PD) and schizophrenia (SCZ) are heritable brain disorders that involve dysregulation of the dopaminergic system. Epidemiological studies have reported potential comorbidity between the disorders, and movement disturbances are common in patients with SCZ before treatment with antipsychotic drugs. Despite this, little is known about shared genetic etiology between the disorders.
Methods: We analyzed recent large genome-wide association studies on patients with SCZ (N = 77,096) and PD (N = 417,508) using a conditional/conjunctional false discovery rate (FDR) approach to evaluate overlap in common genetic variants and improve statistical power for genetic discovery. Using a variety of biological resources, we functionally characterized the identified genomic loci.
Results: We observed genetic enrichment in PD conditional on associations with SCZ and vice versa, indicating polygenic overlap. We then leveraged this cross-trait enrichment using conditional FDR analysis and identified 9 novel PD risk loci and 1 novel SCZ locus at conditional FDR < .01. Furthermore, we identified 9 genomic loci jointly associated with PD and SCZ at conjunctional FDR < .05. There was an even distribution of antagonistic and agonistic effect directions among the shared loci, in line with the insignificant genetic correlation between the disorders. Of 67 genes mapped to the shared loci, 65 are expressed in the human brain and show cell type-specific expression profiles.
Conclusions: Altogether, the study increases understanding of the genetic architectures underlying SCZ and PD, indicating that common molecular genetic mechanisms may contribute to overlapping pathophysiological and clinical features between the disorders.
Keywords: GWAS; Genetic overlap; Parkinson’s disease; RERE; Schizophrenia; ZDHHC2.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
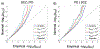
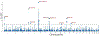
Comment in
-
Do Idiopathic Parkinson's Disease and Schizophrenia Lie on the Same Genetic Spectrum?Biol Psychiatry. 2021 Feb 1;89(3):207-208. doi: 10.1016/j.biopsych.2020.11.011. Biol Psychiatry. 2021. PMID: 33357627 No abstract available.
Similar articles
-
Genome-wide Association Analysis of Schizophrenia and Vitamin D Levels Shows Shared Genetic Architecture and Identifies Novel Risk Loci.Schizophr Bull. 2023 Nov 29;49(6):1654-1664. doi: 10.1093/schbul/sbad063. Schizophr Bull. 2023. PMID: 37163672 Free PMC article.
-
Genome-wide analysis reveals genetic overlap between alcohol use behaviours, schizophrenia and bipolar disorder and identifies novel shared risk loci.Addiction. 2022 Mar;117(3):600-610. doi: 10.1111/add.15680. Epub 2021 Oct 3. Addiction. 2022. PMID: 34472679
-
Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence.Mol Psychiatry. 2020 Apr;25(4):844-853. doi: 10.1038/s41380-018-0332-x. Epub 2019 Jan 4. Mol Psychiatry. 2020. PMID: 30610197 Free PMC article.
-
Discovery of shared genomic loci using the conditional false discovery rate approach.Hum Genet. 2020 Jan;139(1):85-94. doi: 10.1007/s00439-019-02060-2. Epub 2019 Sep 13. Hum Genet. 2020. PMID: 31520123 Review.
-
Genetic comorbidities in Parkinson's disease.Hum Mol Genet. 2014 Feb 1;23(3):831-41. doi: 10.1093/hmg/ddt465. Epub 2013 Sep 20. Hum Mol Genet. 2014. PMID: 24057672 Free PMC article.
Cited by
-
Investigating the shared genetic architecture between depression and subcortical volumes.Nat Commun. 2024 Sep 2;15(1):7647. doi: 10.1038/s41467-024-52121-y. Nat Commun. 2024. PMID: 39223129 Free PMC article.
-
Genome-wide analysis identifies novel shared loci between depression and white matter microstructure.Mol Psychiatry. 2025 Aug;30(8):3455-3465. doi: 10.1038/s41380-025-02932-2. Epub 2025 Feb 19. Mol Psychiatry. 2025. PMID: 39972055 Free PMC article.
-
Genetic and neural mechanisms shared by schizophrenia and depression.Mol Psychiatry. 2025 Sep;30(9):3975-3987. doi: 10.1038/s41380-025-02975-5. Epub 2025 Apr 3. Mol Psychiatry. 2025. PMID: 40175520
-
The Interplay Between Splicing of Two Exon Combinations Differentially Affects Membrane Targeting and Function of Human CaV2.2.Function (Oxf). 2023 Oct 19;5(1):zqad060. doi: 10.1093/function/zqad060. eCollection 2024. Function (Oxf). 2023. PMID: 38020068 Free PMC article.
-
Parkinson's disease and schizophrenia interactomes contain temporally distinct gene clusters underlying comorbid mechanisms and unique disease processes.Schizophrenia (Heidelb). 2024 Feb 27;10(1):26. doi: 10.1038/s41537-024-00439-3. Schizophrenia (Heidelb). 2024. PMID: 38413605 Free PMC article.
References
-
- Lees AJ, Hardy J, Revesz T (2009): Parkinson’s disease. Lancet. 373:2055–2066. - PubMed
-
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA (2012): Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 17:1206–1227. - PubMed
-
- Schapira AHV, Chaudhuri KR, Jenner P (2017): Non-motor features of Parkinson disease. Nat Rev Neurosci. 18:435–450. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

