Disentangling Heterogeneity in Alzheimer's Disease and Related Dementias Using Data-Driven Methods
- PMID: 32201044
- PMCID: PMC7305953
- DOI: 10.1016/j.biopsych.2020.01.016
Disentangling Heterogeneity in Alzheimer's Disease and Related Dementias Using Data-Driven Methods
Abstract
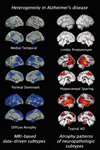
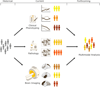
Brain aging is a complex process that includes atrophy, vascular injury, and a variety of age-associated neurodegenerative pathologies, together determining an individual's course of cognitive decline. While Alzheimer's disease and related dementias contribute to the heterogeneity of brain aging, these conditions themselves are also heterogeneous in their clinical presentation, progression, and pattern of neural injury. We reviewed studies that leveraged data-driven approaches to examining heterogeneity in Alzheimer's disease and related dementias, with a principal focus on neuroimaging studies exploring subtypes of regional neurodegeneration patterns. Over the past decade, the steadily increasing wealth of clinical, neuroimaging, and molecular biomarker information collected within large-scale observational cohort studies has allowed for a richer understanding of the variability of disease expression within the aging and Alzheimer's disease and related dementias continuum. Moreover, the availability of these large-scale datasets has supported the development and increasing application of clustering techniques for studying disease heterogeneity in a data-driven manner. In particular, data-driven studies have led to new discoveries of previously unappreciated disease subtypes characterized by distinct neuroimaging patterns of regional neurodegeneration, which are paralleled by heterogeneous profiles of pathological, clinical, and molecular biomarker characteristics. Incorporating these findings into novel frameworks for more differentiated disease stratification holds great promise for improving individualized diagnosis and prognosis of expected clinical progression, and provides opportunities for development of precision medicine approaches for therapeutic intervention. We conclude with an account of the principal challenges associated with data-driven heterogeneity analyses and outline avenues for future developments in the field.
Keywords: Alzheimer’s disease; Brain aging; Clustering; Frontotemporal dementia; Heterogeneity; Lewy body dementias; MRI; Machine learning; Neuroimaging; PET.
Copyright © 2020 Society of Biological Psychiatry. All rights reserved.
Conflict of interest statement
Financial disclosures
Dr. McMillan has received compensation for consulting services from Axon Advisors. Dr. Wolk reports grants from Eli Lilly/Avid, grants from Merck, grants from Biogen, personal fees from GE Healthcare, personal fees from Neuronix Ltd, outside the current work. Dr. Habes, Grothe, Tunc and Davatzikos report no biomedical financial interests or potential conflicts of interest.
Figures
References
-
- Franke K, Ziegler G, Klöppel S, Gaser C (2010): Estimating the age of healthy subjects from T1-weighted {MRI} scans using kernel methods: Exploring the influence of various parameters. NeuroImage 50: 883–892. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

