Sex differences in the response to oxidative and proteolytic stress
- PMID: 32201219
- PMCID: PMC7212483
- DOI: 10.1016/j.redox.2020.101488
Sex differences in the response to oxidative and proteolytic stress
Abstract
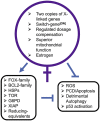
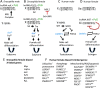
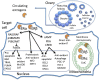
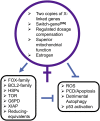
Sex differences in diseases involving oxidative and proteolytic stress are common, including greater ischemic heart disease, Parkinson disease and stroke in men, and greater Alzheimer disease in women. Sex differences are also observed in stress response of cells and tissues, where female cells are generally more resistant to heat and oxidative stress-induced cell death. Studies implicate beneficial effects of estrogen, as well as cell-autonomous effects including superior mitochondrial function and increased expression of stress response genes in female cells relative to male cells. The p53 and forkhead box (FOX)-family genes, heat shock proteins (HSPs), and the apoptosis and autophagy pathways appear particularly important in mediating sex differences in stress response.
Keywords: Heat shock; Oxidative stress; Proteostasis; Sex differences; Sexual antagonistic pleiotropy; Sexual dimorphism.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach.Amino Acids. 2003 Dec;25(3-4):437-44. doi: 10.1007/s00726-003-0048-2. Epub 2003 Nov 7. Amino Acids. 2003. PMID: 14661103 Review.
-
KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease.Free Radic Biol Med. 2018 Feb 1;115:202-218. doi: 10.1016/j.freeradbiomed.2017.11.014. Epub 2017 Nov 21. Free Radic Biol Med. 2018. PMID: 29170092 Free PMC article.
-
Acetyl-L-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1-42-mediated oxidative stress and neurotoxicity: implications for Alzheimer's disease.J Neurosci Res. 2006 Aug 1;84(2):398-408. doi: 10.1002/jnr.20877. J Neurosci Res. 2006. PMID: 16634066
-
The role and therapeutic potential of heat shock proteins in haemorrhagic stroke.J Cell Mol Med. 2019 Sep;23(9):5846-5858. doi: 10.1111/jcmm.14479. Epub 2019 Jul 5. J Cell Mol Med. 2019. PMID: 31273911 Free PMC article. Review.
-
A review of the potential effect of electroacupuncture and moxibustion on cell repair and survival: the role of heat shock proteins.Acupunct Med. 2009 Dec;27(4):183-6. doi: 10.1136/aim.2009.001420. Acupunct Med. 2009. PMID: 19942727 Review.
Cited by
-
Consideration of Sex as a Biological Variable in the Development of Doxorubicin Myotoxicity and the Efficacy of Exercise as a Therapeutic Intervention.Antioxidants (Basel). 2021 Feb 25;10(3):343. doi: 10.3390/antiox10030343. Antioxidants (Basel). 2021. PMID: 33669040 Free PMC article. Review.
-
Selectively advantageous instability in biotic and pre-biotic systems and implications for evolution and aging.Front Aging. 2024 May 16;5:1376060. doi: 10.3389/fragi.2024.1376060. eCollection 2024. Front Aging. 2024. PMID: 38818026 Free PMC article. Review.
-
Selective effects of estradiol on human corneal endothelial cells.bioRxiv [Preprint]. 2023 Apr 28:2023.04.27.538629. doi: 10.1101/2023.04.27.538629. bioRxiv. 2023. Update in: Sci Rep. 2023 Sep 15;13(1):15279. doi: 10.1038/s41598-023-42290-z. PMID: 37162976 Free PMC article. Updated. Preprint.
-
Roadmap for alleviating the manifestations of ageing in the cardiovascular system.Nat Rev Cardiol. 2025 Aug;22(8):577-605. doi: 10.1038/s41569-025-01130-5. Epub 2025 Feb 19. Nat Rev Cardiol. 2025. PMID: 39972009 Review.
-
Impact of Spa Therapy on Symptoms and Quality of Life in Post-COVID-19 Patients with Chronic Conditions.J Clin Med. 2024 Aug 27;13(17):5091. doi: 10.3390/jcm13175091. J Clin Med. 2024. PMID: 39274303 Free PMC article.
References
-
- Alexander H.J., Richardson J.M., Edmands S., Anholt B.R. Sex without sex chromosomes: genetic architecture of multiple loci independently segregating to determine sex ratios in the copepod Tigriopus californicus. J. Evol. Biol. 2015;28:2196–2207. - PubMed
-
- Georges A., Holleley C.E. How does temperature determine sex? Science. 2018;360:601–602. - PubMed
-
- Bashamboo A., McElreavey K. Mechanism of sex determination in humans: insights from disorders of sex development. Sex Dev. 2016;10:313–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

