TIGIT+ A2Ar-Dependent anti-uveitic Treg cells are a novel subset of Tregs associated with resolution of autoimmune uveitis
- PMID: 32201225
- PMCID: PMC7266701
- DOI: 10.1016/j.jaut.2020.102441
TIGIT+ A2Ar-Dependent anti-uveitic Treg cells are a novel subset of Tregs associated with resolution of autoimmune uveitis
Abstract
Regulatory T cells (Tregs) are necessary to prevent autoimmune disease. As such, stable FoxP3 expression is required for the proper function of Tregs in the control of autoimmune disease. Different Treg subsets that utilize different mechanisms of suppression have been identified. The T-cell immunoglobulin immunoreceptor tyrosine-based inhibitory motif (TIGIT) is a relatively new Treg cell marker that has a suppressive function. We have previously identified the adenosine 2A receptor (A2Ar) as a requirement for the emergence of Tregs following resolution of autoimmune disease. Using a FoxP3-GFP-Cre reporter mouse, we identify FoxP3 and 'exFoxP3' cells, show FoxP3 and not exFoxP3 cells are suppressive. We further show FoxP3 cells express TIGIT, and are induced through A2Ar in healthy volunteers, but not patients with autoimmune disease. Furthermore, we show Tregs emerge in the target tissue at the onset of autoimmune disease in an A2Ar-dependent manner. In summary, we identify a novel subset of TIGIT+ Tregs that are induced through stimulation of the A2Ar.
Keywords: A2Ar; Experimental autoimmune uveitis; TIGIT; Tregs.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest None. Statement: All authors concur with the submission and all the material submitted in this manuscript has not been previously reported and is not under consideration for publication elsewhere.
Figures
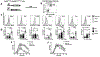
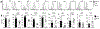

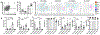
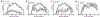


Similar articles
-
A2Ar-dependent PD-1+ and TIGIT+ Treg cells have distinct homing requirements to suppress autoimmune uveitis in mice.Mucosal Immunol. 2023 Aug;16(4):422-431. doi: 10.1016/j.mucimm.2023.04.005. Epub 2023 May 9. Mucosal Immunol. 2023. PMID: 37164238 Free PMC article.
-
Clinical Remission of Sight-Threatening Non-Infectious Uveitis Is Characterized by an Upregulation of Peripheral T-Regulatory Cell Polarized Towards T-bet and TIGIT.Front Immunol. 2018 May 3;9:907. doi: 10.3389/fimmu.2018.00907. eCollection 2018. Front Immunol. 2018. PMID: 29774027 Free PMC article.
-
Retina-specific T regulatory cells bring about resolution and maintain remission of autoimmune uveitis.J Immunol. 2015 Apr 1;194(7):3011-9. doi: 10.4049/jimmunol.1402650. Epub 2015 Feb 25. J Immunol. 2015. PMID: 25716996 Free PMC article.
-
The relationship between TIGIT+ regulatory T cells and autoimmune disease.Int Immunopharmacol. 2020 Jun;83:106378. doi: 10.1016/j.intimp.2020.106378. Epub 2020 Mar 12. Int Immunopharmacol. 2020. PMID: 32172208 Free PMC article. Review.
-
Imbalance of regulatory T cells in human autoimmune diseases.Immunology. 2006 Mar;117(3):289-300. doi: 10.1111/j.1365-2567.2005.02317.x. Immunology. 2006. PMID: 16476048 Free PMC article. Review.
Cited by
-
CD4+ T-Cell Plasticity in Non-Infectious Retinal Inflammatory Disease.Int J Mol Sci. 2021 Sep 3;22(17):9584. doi: 10.3390/ijms22179584. Int J Mol Sci. 2021. PMID: 34502490 Free PMC article. Review.
-
Regulatory T Cells: Therapeutic Opportunities in Uveitis.Front Ophthalmol (Lausanne). 2022 May 25;2:901144. doi: 10.3389/fopht.2022.901144. eCollection 2022. Front Ophthalmol (Lausanne). 2022. PMID: 38983511 Free PMC article. Review.
-
Long-lived autoreactive memory CD4+ T cells mediate the sustained retinopathy in chronic autoimmune uveitis.FASEB J. 2023 Apr;37(4):e22855. doi: 10.1096/fj.202202164R. FASEB J. 2023. PMID: 36906286 Free PMC article.
-
TIGIT as a Promising Therapeutic Target in Autoimmune Diseases.Front Immunol. 2022 Jun 3;13:911919. doi: 10.3389/fimmu.2022.911919. eCollection 2022. Front Immunol. 2022. PMID: 35720417 Free PMC article. Review.
-
Immunosuppressive adenosine-targeted biomaterials for emerging cancer immunotherapy.Front Immunol. 2022 Oct 25;13:1012927. doi: 10.3389/fimmu.2022.1012927. eCollection 2022. Front Immunol. 2022. PMID: 36389700 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

