Role of the ERO1-PDI interaction in oxidative protein folding and disease
- PMID: 32201313
- PMCID: PMC7316501
- DOI: 10.1016/j.pharmthera.2020.107525
Role of the ERO1-PDI interaction in oxidative protein folding and disease
Abstract
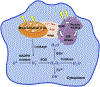
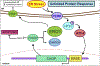
Protein folding in the endoplasmic reticulum is an oxidative process that relies on protein disulfide isomerase (PDI) and endoplasmic reticulum oxidase 1 (ERO1). Over 30% of proteins require the chaperone PDI to promote disulfide bond formation. PDI oxidizes cysteines in nascent polypeptides to form disulfide bonds and can also reduce and isomerize disulfide bonds. ERO1 recycles reduced PDI family member PDIA1 using a FAD cofactor to transfer electrons to oxygen. ERO1 dysfunction critically affects several diseases states. Both ERO1 and PDIA1 are overexpressed in cancers and implicated in diabetes and neurodegenerative diseases. Cancer-associated ERO1 promotes cell migration and invasion. Furthermore, the ERO1-PDIA1 interaction is critical for epithelial-to-mesenchymal transition. Co-expression analysis of ERO1A gene expression in cancer patients demonstrated that ERO1A is significantly upregulated in lung adenocarcinoma (LUAD), glioblastoma and low-grade glioma (GBMLGG), pancreatic ductal adenocarcinoma (PAAD), and kidney renal papillary cell carcinoma (KIRP) cancers. ERO1Α knockdown gene signature correlates with knockdown of cancer signaling proteins including IGF1R, supporting the search for novel, selective ERO1 inhibitors for the treatment of cancer. In this review, we explore the functions of ERO1 and PDI to support inhibition of this interaction in cancer and other diseases.
Keywords: Cancer; Endoplasmic reticulum Oxidase; Gene expression; Protein disulfide Isomerase; Protein folding; Targeted therapy.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that there are no conflicts of interest.
Figures

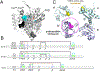
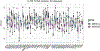



Similar articles
-
A PDI-catalyzed thiol-disulfide switch regulates the production of hydrogen peroxide by human Ero1.Free Radic Biol Med. 2015 Jun;83:361-72. doi: 10.1016/j.freeradbiomed.2015.02.011. Epub 2015 Feb 17. Free Radic Biol Med. 2015. PMID: 25697776
-
Ero1-PDI interactions, the response to redox flux and the implications for disulfide bond formation in the mammalian endoplasmic reticulum.Philos Trans R Soc Lond B Biol Sci. 2013 Mar 25;368(1617):20110403. doi: 10.1098/rstb.2011.0403. Print 2013 May 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23530257 Free PMC article.
-
The endoplasmic reticulum sulfhydryl oxidase Ero1β drives efficient oxidative protein folding with loose regulation.Biochem J. 2011 Feb 15;434(1):113-21. doi: 10.1042/BJ20101357. Biochem J. 2011. PMID: 21091435
-
Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum.Antioxid Redox Signal. 2010 Oct;13(8):1177-87. doi: 10.1089/ars.2010.3230. Antioxid Redox Signal. 2010. PMID: 20486761 Review.
-
ERO1-PDI Redox Signaling in Health and Disease.Antioxid Redox Signal. 2021 Nov 1;35(13):1093-1115. doi: 10.1089/ars.2021.0018. Epub 2021 Jul 13. Antioxid Redox Signal. 2021. PMID: 34074138 Free PMC article. Review.
Cited by
-
Biomarkers of autoimmunity and beta cell metabolism in type 1 diabetes.Front Immunol. 2022 Oct 27;13:1028130. doi: 10.3389/fimmu.2022.1028130. eCollection 2022. Front Immunol. 2022. PMID: 36389721 Free PMC article. Review.
-
Improved Production of Recombinant Carboxylesterase FumDM by Co-Expressing Molecular Chaperones in Pichia pastoris.Toxins (Basel). 2023 Feb 14;15(2):156. doi: 10.3390/toxins15020156. Toxins (Basel). 2023. PMID: 36828470 Free PMC article.
-
Identification of the H3K36me3 reader LEDGF/p75 in the pancancer landscape and functional exploration in clear cell renal cell carcinoma.Comput Struct Biotechnol J. 2023 Aug 26;21:4134-4148. doi: 10.1016/j.csbj.2023.08.023. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37675289 Free PMC article.
-
Intracellular Sources of ROS/H2O2 in Health and Neurodegeneration: Spotlight on Endoplasmic Reticulum.Cells. 2021 Jan 25;10(2):233. doi: 10.3390/cells10020233. Cells. 2021. PMID: 33504070 Free PMC article. Review.
-
Endoplasmic Reticulum Stress in Bronchopulmonary Dysplasia: Contributor or Consequence?Cells. 2024 Oct 26;13(21):1774. doi: 10.3390/cells13211774. Cells. 2024. PMID: 39513884 Free PMC article. Review.
References
-
- Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, … Sitia R (2012). Ero1α regulates Ca2 fluxes at the endoplasmic reticulum–mitochondria interface (MAM). Antioxidants & Redox Signaling, 16, 1077–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

