Prominins control ciliary length throughout the animal kingdom: New lessons from human prominin-1 and zebrafish prominin-3
- PMID: 32201384
- PMCID: PMC7196652
- DOI: 10.1074/jbc.RA119.011253
Prominins control ciliary length throughout the animal kingdom: New lessons from human prominin-1 and zebrafish prominin-3
Abstract
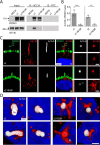
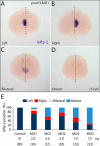
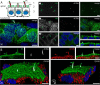
Prominins (proms) are transmembrane glycoproteins conserved throughout the animal kingdom. They are associated with plasma membrane protrusions, such as primary cilia, as well as extracellular vesicles derived thereof. Primary cilia host numerous signaling pathways affected in diseases known as ciliopathies. Human PROM1 (CD133) is detected in both somatic and cancer stem cells and is also expressed in terminally differentiated epithelial and photoreceptor cells. Genetic mutations in the PROM1 gene result in retinal degeneration by impairing the proper formation of the outer segment of photoreceptors, a modified cilium. Here, we investigated the impact of proms on two distinct examples of ciliogenesis. First, we demonstrate that the overexpression of a dominant-negative mutant variant of human PROM1 (i.e. mutation Y819F/Y828F) significantly decreases ciliary length in Madin-Darby canine kidney cells. These results contrast strongly to the previously observed enhancing effect of WT PROM1 on ciliary length. Mechanistically, the mutation impeded the interaction of PROM1 with ADP-ribosylation factor-like protein 13B, a key regulator of ciliary length. Second, we observed that in vivo knockdown of prom3 in zebrafish alters the number and length of monocilia in the Kupffer's vesicle, resulting in molecular and anatomical defects in the left-right asymmetry. These distinct loss-of-function approaches in two biological systems reveal that prom proteins are critical for the integrity and function of cilia. Our data provide new insights into ciliogenesis and might be of particular interest for investigations of the etiologies of ciliopathies.
Keywords: ADP-ribosylation factor (ARF); ADP-ribosylation factor-like protein 13B (Arl13b); CD133; Kupffer's vesicle; MDCK; cilia; ciliopathy; cilium; development; left-right asymmetry; membrane polarity; membrane protein; prominin-1 (PROM1); zebrafish.
© 2020 Jászai et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Weigmann A., Corbeil D., Hellwig A., and Huttner W. B. (1997) Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 94, 12425–12430 10.1073/pnas.94.23.12425 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

