Membrane binding proteins of coronaviruses
- PMID: 32201500
- PMCID: PMC7079996
- DOI: 10.2217/fvl-2018-0144
Membrane binding proteins of coronaviruses
Abstract
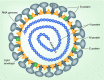
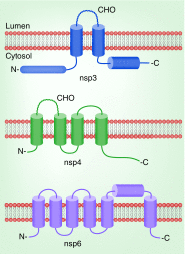
Coronaviruses (CoVs) infect many species causing a variety of diseases with a range of severities. Their members include zoonotic viruses with pandemic potential where therapeutic options are currently limited. Despite this diversity CoVs share some common features including the production, in infected cells, of elaborate membrane structures. Membranes represent both an obstacle and aid to CoV replication - and in consequence - virus-encoded structural and nonstructural proteins have membrane-binding properties. The structural proteins encounter cellular membranes at both entry and exit of the virus while the nonstructural proteins reorganize cellular membranes to benefit virus replication. Here, the role of each protein in membrane binding is described to provide a comprehensive picture of their role in the CoV replication cycle.
Keywords: bending; coronavirus; egress; fusion; membrane; peptide; replication; web.
© 2019 Ian M Jones.
Conflict of interest statement
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Figures

References
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. - PubMed
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
