FRAT1 Enhances the Proliferation and Tumorigenesis of CD133+Nestin+ Glioma Stem Cells In Vitro and In Vivo
- PMID: 32201513
- PMCID: PMC7066019
- DOI: 10.7150/jca.37622
FRAT1 Enhances the Proliferation and Tumorigenesis of CD133+Nestin+ Glioma Stem Cells In Vitro and In Vivo
Abstract
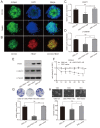
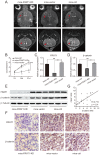
Glioma stem cells (GSCs) are considered the source for development, recurrence, and poor prognosis of glioma, so treatment targeted GSCs is of great interest. The frequently rearranged in advanced T cell lymphomas-1 (FRAT1) gene is an important member of the Wnt/β-catenin signaling transduction pathway, and aberrantly activation of Wnt signaling has been identified to contribute to the tumorigenesis, proliferation, invasion of a variety kinds of cancer stem cells. However, correlations between FRAT1 and GSCs and the specific mechanisms remain unclear. In this study, we aimed to investigate the effect of FRAT1 on GSCs proliferation, colony formation, sphere formation and tumorigenesity in vitro and in vivo and its underlying mechanism. Lentiviral transfection was used to construct GSCs with low FRAT1 expression. The expression of FRAT1 on GSCs proliferation in vitro was assessed by cell counting kit-8(CCK-8). Colony formation and sphere formation assays were conducted to assess the colony and sphere formation ability of GSCs. Then, an intracranial glioma nude mouse model was built to measure the effect of low FRAT1 expression on GSCs proliferation and tumorigenesity in vivo. Real-time PCR, Western blot, and Immunohistochemistry were processed to detect the mRNA and protein expressions of FRAT1, β-catenin in the glioma tissue of xenograft mice to study their correlations. The functional assays verifed that low FRAT1 expression inhibited CD133+Nestin+ GSCs proliferation, colony formation, sphere formation ability in vitro. In vivo GSCs xenograft mice model showed that low FRAT1 expression suppressed the proliferation and tumorigenesity of CD133+Nestin+ GSCs and reduced β-catenin mRNA and protein expression. Furthermore, the expression of FRAT1 and β-catenin were positively correlated. Altogether, results indicate that FRAT1 enhances the proliferation, colony formation, sphere formation and tumorigenesity of CD133+Nestin+ glioma stem cells in vitro and in vivo as well as the expression of β-catenin. Therefore, inhibiting proliferation of GSCs and FRAT1 may be a molecular target to GSCs in treating human glioma in the future.
Keywords: Glioma stem cells (GSCs); Proliferation; The frequently rearranged in advanced T cell lymphomas-1(FRAT1); Wnt/β-catenin pathway.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–38. - PubMed
-
- Allegra A, Alonci A, Penna G, Innao V, Gerace D, Rotondo F. et al. The cancer stem cell hypothesis: a guide to potential molecular targets. Cancer Invest. 2014;32:470–95. - PubMed
-
- Imaizumi A, Onishi H, Yamasaki A, Kawamoto M, Umebayashi M, Morisaki T. et al. Hypoxic Conditions Promote Gemcitabine Sensitivity in a Pancreatic Cancer Stem Cell Line. Anticancer Res. 2016;36:653–7. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

