DRG Neurons Promote Perineural Invasion of Endometrial Cancer via GluR2
- PMID: 32201522
- PMCID: PMC7066017
- DOI: 10.7150/jca.40055
DRG Neurons Promote Perineural Invasion of Endometrial Cancer via GluR2
Abstract
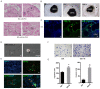
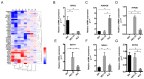
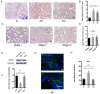
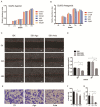
Background: Perineural invasion (PNI) is correlated with negative prognosis in multiple cancers, but its role in endometrial cancer (EC) is still largely unknown; thus, targeted treatment for nerve infiltration is lacking as well. Methods: The interaction between nerve and EC cells were investigated by in vitro neural invasion assay and transwell coculture system. Then the nerve-related receptor gene glutamate ionotropic receptor AMPA type subunit 2 (GRIA2) was detected in EC tissues and cells using PCR array, western blotting, and immunohistochemistry. The role of GluR2 (gene name GRIA2) on EC proliferation, migration and invasion was evaluated by a GluR2 antagonist and shRNA. At the same time, the neurotransmitter effect on GluR2 (glutamate) from the cocultured conditional medium was measured using high-performance liquid chromatography (HPLC). Results: EC cell line Ishikawa (ISK) showed the ability to migrate along neurites in vitro and the numbers of migrated/invaded EC cells in the DRG neuron coculture group were significantly increased. The expression of GluR2 in EC tissue was found to be higher than that in para-carcinoma tissue. After GluR2 antagonist and GluR2 shRNA treatment, the proliferation, migration and invasion of ISK cells was markedly inhibited. Moreover, the ability of DRG neurons to promote the migration and invasion of ISK cells could also be attenuated by downregulation of GluR2, and the concentration of the neurotransmitter glutamate was notably increased in the coculture conditional medium compared to that in the DRG neuron or ISK cells alone. Conclusions: DRG neurons promote metastasis of EC cells via GluR2, which might be a risk factor for PNI in EC. Moreover, the perineural system may promote tumor invasion and metastasis under certain circumstances.
Keywords: DRG neurons; GluR2; endometrial cancer; metastasis; perineural invasion.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
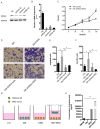
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA cancer. J. Clin. 2017;67:7–30. - PubMed
-
- Morice P, Leary A, Creutzberg C. et al. Endometrial cancer. Lancet. 2016;387:1094–1108. - PubMed
-
- Fleming GF, Brunetto VL, Cella D. et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–2166. - PubMed
-
- Sutton G, Axelrod JH, Bundy BN. et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. 2005;97:755–763. - PubMed
-
- Jobling P, Pundavela J, Oliveira SM. et al. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015;75:1777–1781. - PubMed
LinkOut - more resources
Full Text Sources

