A seven-year surveillance of epidemiology of malaria reveals travel and gender are the key drivers of dispersion of drug resistant genotypes in Kenya
- PMID: 32201636
- PMCID: PMC7073242
- DOI: 10.7717/peerj.8082
A seven-year surveillance of epidemiology of malaria reveals travel and gender are the key drivers of dispersion of drug resistant genotypes in Kenya
Abstract
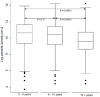
Malaria drug resistance is a global public health concern. Though parasite mutations have been associated with resistance, other factors could influence the resistance. A robust surveillance system is required to monitor and help contain the resistance. This study established the role of travel and gender in dispersion of chloroquine resistant genotypes in malaria epidemic zones in Kenya. A total of 1,776 individuals presenting with uncomplicated malaria at hospitals selected from four malaria transmission zones in Kenya between 2008 and 2014 were enrolled in a prospective surveillance study assessing the epidemiology of malaria drug resistance patterns. Demographic and clinical information per individual was obtained using a structured questionnaire. Further, 2 mL of blood was collected for malaria diagnosis, parasitemia quantification and molecular analysis. DNA extracted from dried blood spots collected from each of the individuals was genotyped for polymorphisms in Plasmodium falciparum chloroquine transporter gene (Pfcrt 76), Plasmodium falciparum multidrug resistant gene 1 (Pfmdr1 86 and Pfmdr1 184) regions that are putative drug resistance genes using both conventional polymerase chain reaction (PCR) and real-time PCR. The molecular and demographic data was analyzed using Stata version 13 (College Station, TX: StataCorp LP) while mapping of cases at the selected geographic zones was done in QGIS version 2.18. Chloroquine resistant (CQR) genotypes across gender revealed an association with chloroquine resistance by both univariate model (p = 0.027) and by multivariate model (p = 0.025), female as reference group in both models. Prior treatment with antimalarial drugs within the last 6 weeks before enrollment was associated with carriage of CQR genotype by multivariate model (p = 0.034). Further, a significant relationship was observed between travel and CQR carriage both by univariate model (p = 0.001) and multivariate model (p = 0.002). These findings suggest that gender and travel are significantly associated with chloroquine resistance. From a gender perspective, males are more likely to harbor resistant strains than females hence involved in strain dispersion. On the other hand, travel underscores the role of transport network in introducing spread of resistant genotypes, bringing in to focus the need to monitor gene flow and establish strategies to minimize the introduction of resistance strains by controlling malaria among frequent transporters.
Keywords: Chloroquine; Drug resistance; Gender; Malaria; Plasmodium falciparum; Travel.
©2020 Maraka et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures




References
-
- Achieng AO, Muiruri P, Ingasia LA, Opot BH, Juma DW, Yeda R, Ngalah B, Ogutu B, Andagalu B, Akala H, Kamau E. Temporal trends in prevalence of Plasmodium falciparum molecular markers selected for by artemether- lumefantrine treatment in pre- ACT and post-ACT parasites in western Kenya. International Journal for Parasitology: Drugs and Drug Resistance. 2015;5(3):92–99. doi: 10.1016/j.ijpddr.2015.05.005. - DOI - PMC - PubMed
-
- Akala HM, Achieng AO, Eyase FL, Juma DW, Ingasia L, Cheruiyot AC, Okello C, Omariba D, Owiti EA, Muriuki C, Yeda R, Andagalu B, Johnson JD, Kamau E. Five-year tracking of plasmodium falciparum allele frequencies in a holoendemic area with indistinct seasonal transitions. Journal of Multidisciplinary Healthcare. 2014;7:515–523. doi: 10.2147/JMDH.S67252. - DOI - PMC - PubMed
-
- Amambua-Ngwa A, Amenga-Etego L, Kamau E, Amato R, Ghansah A, Golassa L, Randrianarivelojosia M, Ishengoma D, Apinjoh T, Maïga-Ascofaré O, Andagalu B, Yavo W, Bouyou-Akotet M, Kolapo O, Mane K, Worwui A, Jeffries D, Simpson V, D’Alessandro U, Kwiatkowski D, Djimde AA. Major subpopulations of Plasmodium falciparum in sub-Saharan Africa. Science. 2019;365(6455):813–816. doi: 10.1126/science.aav5427. - DOI - PubMed
-
- Angira CHO, Otieno OA, Muga RO, Abong’o BO. Factors contributing to antimalarial drug resistance in Rachuonyo district, Kenya. East African Journal of Public Health. 2010;7(1):11–15. - PubMed
LinkOut - more resources
Full Text Sources

