Codon optimization, expression in Escherichia coli, and immunogenicity analysis of deformed wing virus (DWV) structural protein
- PMID: 32201647
- PMCID: PMC7071823
- DOI: 10.7717/peerj.8750
Codon optimization, expression in Escherichia coli, and immunogenicity analysis of deformed wing virus (DWV) structural protein
Abstract
Background: Deformed wing virus (DWV) is a serious threat to honey bees (Apis mellifera) and is considered a major cause of elevated losses of honey bee colonies. However, lack of information on the immunogenicity of DWV structural proteins has hindered the development of effective biocontrol drugs.
Methods: We optimized the VP1, VP2 and VP3 codons of DWV surface capsid protein genes on the basis of an Escherichia coli codon bias, and the optimized genes of roVP1, roVP2 and roVP3 were separately expressed in E. coli and purified. Next, the three recombinant proteins of roVP1, roVP2 and roVP3 were intramuscularly injected into BALB/c and the immunogenicity was evaluated by the levels of specific IgG and cytokines. Furthermore, anti-roVP-antisera (roVP1 or roVP2 or roVP3) from the immunized mice was incubated with DWV for injecting healthy white-eyed pupae for the viral challenge test, respectively.
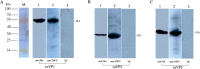
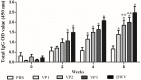
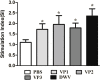
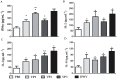
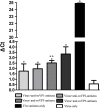
Results: The optimized genes roVP1, roVP2 and roVP3 achieved the expression in E. coli using SDS-PAGE and Western blotting. Post-immunization, roVP2 and roVP3 exhibited higher immunogenicity than roVP1 and stimulated a stronger humoral immune response in the mice, which showed that the recombinant proteins of roVP3 and roVP2 induced a specific immune response in the mice. In the challenge test, data regarding quantitative real-time RT-PCR (qRT-PCR) from challenged pupae showed that the level of virus copies in the recombinant protein groups was significantly lower than that of the virus-only group at 96 h post-inoculation (P < 0.05). Among them, the degree of neutralization using antibodies raised to the recombinant proteins are between approximately 2-fold and 4-fold and the virus copies of the roVP3 group are the lowest in the three recombinant protein groups, which indicated that specific antibodies against recombinant proteins roVP1, roVP2 and roVP3 of DWV could neutralize DWV to reduce the virus titer in the pupae. Collectively, these results demonstrated that the surface capsid protein of DWV acted as candidates for the development of therapeutic antibodies against the virus.
Keywords: Challenge test; Codon optimization; DWV; Immune responses; Structural proteins.
© 2020 Fei et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Bailey L, Ball BV. Honey bee pathology. Second Edition. London: Academic Press; 1991.
-
- Benaets K, Van Geystelen A, Cardoen D, De Smet L, De Graaf DC, Schoofs L, Wenseleers T. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proceedings of the Royal Society B: Biological Sciences. 2017;284(1484):2149. doi: 10.1098/rspb.2016.2149. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

