Interventions to improve quantitative measures of parent satisfaction in neonatal care: a systematic review
- PMID: 32201743
- PMCID: PMC7073789
- DOI: 10.1136/bmjpo-2019-000613
Interventions to improve quantitative measures of parent satisfaction in neonatal care: a systematic review
Abstract
Objective: Interventions improving parent satisfaction can reduce parent stress, may improve parent-infant bonding and infant outcomes. Our objective was to systematically review neonatal interventions relating to parents of infants of all gestations where an outcome was parent satisfaction.
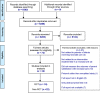
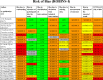
Methods: We searched the databases MEDLINE, EMBASE, PsychINFO, Cochrane Central Register of Controlled Trials, CINAHL, HMIC, Maternity and Infant Care between 1 January 1946 and 1 October 2017. Inclusion criteria were randomised controlled trials (RCT), cohort studies and other non-randomised studies if participants were parents of infants receiving neonatal care, interventions were implemented in neonatal units (of any care level) and ≥1 quantitative outcome of parent satisfaction was measured. Included studies were limited to the English language only. We extracted study characteristics, interventions, outcomes and parent involvement in intervention design. Included studies were not sufficiently homogenous to enable quantitative synthesis. We assessed quality with the Cochrane Collaboration risk of bias tool (randomised) and the ROBINS-I tool (Risk Of Bias In Non-randomised Studies - of Interventions) (non-randomised studies).
Results: We identified 32 studies with satisfaction measures from over 2800 parents and grouped interventions into 5 themes. Most studies were non-randomised involving preterm infants. Parent satisfaction was measured by 334 different questions in 29 questionnaires (only 6/29 fully validated). 18/32 studies reported higher parent satisfaction in the intervention group. The intervention theme with most studies reporting higher satisfaction was parent involvement (10/14). Five (5/32) studies reported involving parents in intervention design. All studies had high risk of bias.
Conclusions: Many interventions, commonly relating to parent involvement, are reported to improve parent satisfaction. Inconsistency in satisfaction measurements and high risk of bias makes this low-quality evidence. Standardised, validated parent satisfaction measures are needed, as well as higher quality trials of parent experience involving parents in intervention design.
Prospero registration number: CRD42017072388.
Keywords: neonatology; outcomes research; patient perspective.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: SS has received research grants from the National Institute of Health Research (NIHR), the NIHR CLAHRC NWL, Rosetrees Trust and CW+ charity. NM is Director of the Neonatal Data Analysis Unit at Imperial College London. In the last 5 years, NM has served on the Board of Trustees of the Royal College of Paediatrics and Child Health, David Harvey Trust, Medical Women’s Federation and Medact; and is a member of the Nestle Scientific Advisory Board. NM has received research grants from the British Heart Foundation, Medical Research Council, National Institute of Health Research, Westminster Research Fund, Collaboration for Leadership in Applied Health and Care Northwest London, Healthcare Quality Improvement Partnership, Bliss, Prolacta Life Sciences, Chiesi, Shire and HCA International; travel and accommodation expenses from Nutricia, Prolacta, Nestle and Chiesi; honoraria from Ferring Pharmaceuticals and Alexion Pharmaceuticals for contributions to expert advisory boards and Chiesi for contributing to a lecture programme. CG is funded by the UK Medical Research Council (MRC) through a Clinician Scientist Fellowship award. He has received support from Chiesi Pharmaceuticals to attend an educational conference; in the past 5 years, he has been investigator on received research grants from Medical Research Council, National Institute of Health Research, Canadian Institute of Health Research, Department of Health in England, Mason Medical Research Foundation, Westminster Medical School Research Trust and Chiesi Pharmaceuticals.
Figures

References
-
- Neonatal Data Analysis Unit . Neonatal data analysis unit annual report 2017, 2018. Available: https://www.rcpch.ac.uk/sites/default/files/2018-10/2018_nnap_report_on_...
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
