Metallotexaphyrins as MRI-Active Catalytic Antioxidants for Neurodegenerative Disease: A Study on Alzheimer's Disease
- PMID: 32201749
- PMCID: PMC7074011
- DOI: 10.1016/j.chempr.2019.12.016
Metallotexaphyrins as MRI-Active Catalytic Antioxidants for Neurodegenerative Disease: A Study on Alzheimer's Disease
Abstract
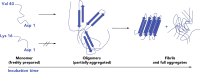
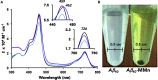
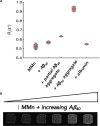
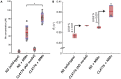
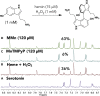
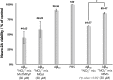
The complex etiology of neurodegeneration continues to stifle efforts to develop effective therapeutics. New agents elucidating key pathways causing neurodegeneration might serve to increase our understanding and potentially lead to improved treatments. Here, we demonstrate that a water-soluble manganese(II) texaphyrin (MMn) is a suitable magnetic resonance imaging (MRI) contrast agent for detecting larger amyloid beta constructs. The imaging potential of MMn was inferred on the basis of in vitro studies and in vivo detection in Alzheimer's disease C. elegans models via MRI and ICP-MS. In vitro antioxidant- and cellular-based assays provide support for the notion that this porphyrin analog shows promise as a therapeutic agent able to mitigate the oxidative and nitrative toxic effects considered causal in neurodegeneration. The present report marks the first elaboration of an MRI-active metalloantioxidant that confers diagnostic and therapeutic benefit in Alzheimer's disease models without conjugation of a radioisotope, targeting moiety, or therapeutic payload.
Keywords: Alzheimer’s disease; C. elegans AD models; MRI; amyloid aggregation; amyloid beta; amyloid beta modifications by ROS and RNS; expanded porphyrin; metalloantioxidant; neurodegeneration; texaphyrin.
© 2019 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

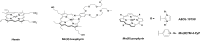



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
