Study on the Assembly Structure Variation of Cetyltrimethylammonium Bromide on the Surface of Gold Nanoparticles
- PMID: 32201780
- PMCID: PMC7081447
- DOI: 10.1021/acsomega.9b03823
Study on the Assembly Structure Variation of Cetyltrimethylammonium Bromide on the Surface of Gold Nanoparticles
Abstract
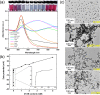
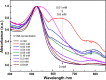
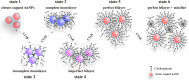
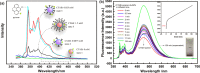
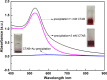
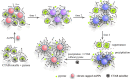
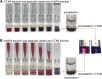
In this work, the self-assembly behavior of cetyltrimethylammonium bromide (CTAB) on the surface of citrate-capped gold nanoparticles (AuNPs) in solution has been studied by UV-vis absorption spectroscopy, fluorescence probe techniques, ζ potentiometric methods, transmission electron microscopy, etc. The UV-vis spectra show that the color with the increase of CTAB for the mixture containing CTAB and a given amount of AuNPs changes from red to blue and then to red. The absolute value of ζ potential corresponding to this color change decreases initially and then increases. Specially, the reversible color change, from red to blue and then to red, could be observed only in the case of a gradual addition of a AuNP solution to a CTAB solution; however, this reversible change is not suitable for the mixture formed in a reverse order of mixing. The results from pyrene used as the fluorescence probe indicate that the features in the fluorescence spectrum (including fluorescence quenching, I 1/I 3, and the excimer) well correspond to those from the UV-vis spectrum mentioned above. Based on the experimental results, the mechanism of the assembly structure variation of CTAB on the surface of negatively charged AuNPs was proposed. For a given amount of AuNPs, the assembly structure of CTAB on the surface of AuNPs undergoes the transformation from a monolayer to a bilayer with the increase of CTAB. In the case of the concentration of CTAB far beyond its critical micelle concentration (CMC) and the higher ratio of CTAB and AuNPs, there is a possibility of the formation of an extra micellar structure only after the formation of a double-layer structure.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Wang Z. L.; Mohamed M. B.; Link S.; El-Sayed M. A. Crystallographic Facets and Shapes of Gold Nanorods of Different Aspect Ratios. Surf. Sci. 1999, 440, L809–L814. 10.1016/S0039-6028(99)00865-1. - DOI
-
- Nikoobakht B.; El-Sayed M. A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. 10.1021/cm020732l. - DOI
-
- Nikoobakht B.; El-Sayed M. A. Evidence for Bilayer Assembly of Cationic Surfactants on the Surface of Gold Nanorods. Langmuir 2001, 17, 6368–6374. 10.1021/la010530o. - DOI
LinkOut - more resources
Full Text Sources
