Facile Synthesis of Luffa Sponge Activated Carbon Fiber Based Carbon Quantum Dots with Green Fluorescence and Their Application in Cr(VI) Determination
- PMID: 32201847
- PMCID: PMC7081637
- DOI: 10.1021/acsomega.0c00195
Facile Synthesis of Luffa Sponge Activated Carbon Fiber Based Carbon Quantum Dots with Green Fluorescence and Their Application in Cr(VI) Determination
Abstract
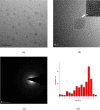
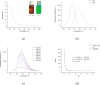
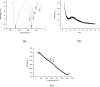
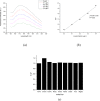
Carbon quantum dots (CQDs) were prepared by a chemical oxidation method using luffa sponge based activated carbon fiber as the raw material. The obtained CQDs were well characterized. The fluorescence quenching effect of Cr(VI) ion on CQDs was investigated. The results show that the addition of Cr(VI) changes the intensity of the ultraviolet characteristic absorption peak of CQDs, and causes static quenching of the fluorescence of CQDs. With the increase in the Cr(VI) concentration, the fluorescence of CQDs was gradually extinguished linearly.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Fu Z.; Chen W.; Jiang Z.; Macdonald B. E.; Lin Y.; Fei C.; Zhang L.; Lavernia E. J. Influence of Cr removal on the microstructure and mechanical behaviour of a high-entropy Al0.8Ti0.2CoNiFeCr alloy fabricated by powder metallurgy. Powder Metall. 2018, 106–114. 10.1080/00325899.2018.1427662. - DOI
-
- Coşkun R.; Birgül H.; Delibaş A. Synthesis of functionalized PET fibers by grafting and modification and their application for Cr(VI) ion removal. J. Polym. Res. 2018, 25, 29. 10.1007/s10965-017-1429-7. - DOI
-
- Feng X.; Liang C.; Yu J.; Jiang X. Facile fabrication of graphene oxide-polyethylenimine composite and its application for the Cr(VI) removal. Sep. Sci. Technol. 2018, 53, 2376–2387. 10.1080/01496395.2018.1458880. - DOI
LinkOut - more resources
Full Text Sources

