Arabidopsis ECHIDNA protein is involved in seed coloration, protein trafficking to vacuoles, and vacuolar biogenesis
- PMID: 32201898
- PMCID: PMC7475254
- DOI: 10.1093/jxb/eraa147
Arabidopsis ECHIDNA protein is involved in seed coloration, protein trafficking to vacuoles, and vacuolar biogenesis
Abstract
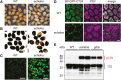
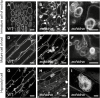
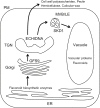
Flavonoids are a major group of plant-specific metabolites that determine flower and seed coloration. In plant cells, flavonoids are synthesized at the cytosolic surface of the endoplasmic reticulum and are sequestered in the vacuole. It is possible that membrane trafficking, including vesicle trafficking and organelle dynamics, contributes to flavonoid transport and accumulation. However, the underlying mechanism has yet to be fully elucidated. Here we show that the Arabidopsis ECHIDNA protein plays a role in flavonoid accumulation in the vacuole and protein trafficking to the vacuole. We found defective pigmentation patterns in echidna seed, possibly caused by reduced levels of proanthocyanidins, which determine seed coloration. The echidna mutant has defects in protein sorting to the protein storage vacuole as well as vacuole morphology. These findings indicate that ECHIDNA is involved in the vacuolar trafficking pathway as well as the previously described secretory pathway. In addition, we found a genetic interaction between echidna and green fluorescent seed 9 (gfs9), a membrane trafficking factor involved in flavonoid accumulation. Our findings suggest that vacuolar trafficking and/or vacuolar development, both of which are collectively regulated by ECHIDNA and GFS9, are required for flavonoid accumulation, resulting in seed coat pigmentation.
Keywords: Arabidopsis thaliana; trans-Golgi network; ECHIDNA; GREEN FLUORESCENT SEED 9; mucilage; protein sorting; seed coloration; vacuolar morphology; vacuolar trafficking; vacuole.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Experimental Biology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Abrahams S, Lee E, Walker AR, Tanner GJ, Larkin PJ, Ashton AR. 2003. The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. The Plant Journal 35, 624–636. - PubMed
-
- Appelhagen I, Nordholt N, Seidel T, Spelt K, Koes R, Quattrochio F, Sagasser M, Weisshaar B. 2015. TRANSPARENT TESTA 13 is a tonoplast P3A-ATPase required for vacuolar deposition of proanthocyanidins in Arabidopsis thaliana seeds. The Plant Journal 82, 840–849. - PubMed
-
- Arganda-Carreras I, Kaynig V, Rueden C, Eliceiri KW, Schindelin J, Cardona A, Sebastian Seung H. 2017. Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 33, 2424–2426. - PubMed
-
- Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF. 2005. A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 102, 2649–2654. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

