Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It's Time to Sound the Alarm
- PMID: 32202088
- PMCID: PMC7308241
- DOI: 10.5534/wjmh.200012
Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It's Time to Sound the Alarm
Abstract
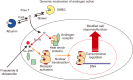
5α-dihydrotestosterone (5α-DHT) is the most potent natural androgen. 5α-DHT elicits a multitude of physiological actions, in a host of tissues, including prostate, seminal vesicles, hair follicles, skin, kidney, and lacrimal and meibomian glands. However, the physiological role of 5α-DHT in human physiology, remains questionable and, at best, poorly appreciated. Recent emerging literature supports a role for 5α-DHT in the physiological function of liver, pancreatic β-cell function and survival, ocular function and prevention of dry eye disease and kidney physiological function. Thus, inhibition of 5α-reductases with finasteride or dutasteride to reduce 5α-DHT biosynthesis in the course of treatment of benign prostatic hyperplasia (BPH) or male pattern hair loss, known as androgenetic alopecia (AGA) my induces a novel form of tissue specific androgen deficiency and contributes to a host of pathophysiological conditions, that are yet to be fully recognized. Here, we advance the concept that blockade of 5α-reductases by finasteride or dutasteride in a mechanism-based, irreversible, inhabitation of 5α-DHT biosynthesis results in a novel state of androgen deficiency, independent of circulating testosterone levels. Finasteride and dutasteride are frequently prescribed for long-term treatment of lower urinary tract symptoms in men with BPH and in men with AGA. This treatment may result in development of non-alcoholic fatty liver diseases (NAFLD), insulin resistance (IR), type 2 diabetes (T2DM), dry eye disease, potential kidney dysfunction, among other metabolic dysfunctions. We suggest that long-term use of finasteride and dutasteride may be associated with health risks including NAFLD, IR, T2DM, dry eye disease and potential kidney disease.
Keywords: 5-alpha reductase inhibitors; Diabetes mellitus; Dry eye syndromes; Hypogonadism; Kidney diseases; Non-alcoholic fatty liver disease.
Copyright © 2020 Korean Society for Sexual Medicine and Andrology.
Conflict of interest statement
The author has nothing to disclose.
Figures


Comment in
-
Bone Health Risks Associated with Finasteride and Dutasteride Long-Term Use.World J Mens Health. 2021 Apr;39(2):389-390. doi: 10.5534/wjmh.200138. Epub 2020 Nov 26. World J Mens Health. 2021. PMID: 33663032 Free PMC article. No abstract available.
References
-
- Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M. Adverse effects of 5α-reductase inhibitors: What do we know, don't know, and need to know? Rev Endocr Metab Disord. 2015;16:177–198. - PubMed
-
- Traish AM. The post-finasteride syndrome: clinical manifestation of drug-induced epigenetics due to endocrine disruption. Curr Sex Health Rep. 2018;10:88–103.
-
- Traish AM. 5α-reductases in human physiology: an unfolding story. Endocr Pract. 2012;18:965–975. - PubMed
-
- Traish AM, Guay AT, Zitzmann M. 5α-Reductase inhibitors alter steroid metabolism and may contribute to insulin resistance, diabetes, metabolic syndrome and vascular disease: a medical hypothesis. Horm Mol Biol Clin Investig. 2014;20:73–80. - PubMed
-
- Traish AM. Negative impact of testosterone deficiency and 5α-reductase inhibitors therapy on metabolic and sexual function in men. Adv Exp Med Biol. 2017;1043:473–526. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

